Main Content
Cell signalling and cell rearrangement during organ morphogenesis
The organization of body pattern in developing multi-cellular organisms is controlled to a large extent by cell-cell signaling. In the past two decades, the molecular components of a relatively small number of diverse developmental signaling cascades conserved throughout evolution have been identified. We have been studying two important developmental signals (Dpp/BMP and Fgf), and our efforts concentrated firstly on characterizing the signaling pathways in detail and deciphering their molecular logic, and secondly on understanding how these pathways control exquisite cellular behavior during development, both in Drosophila and in zebrafish. Our most intense research efforts are directed towards a profound understanding of cell behavior in branching morphogenesis, a process that leads to the ramification of epithelial structures such as seen in the lung, the kidney, many internal glands as well as the vascular system.
Cell signaling in organ formation
It has been proposed more than a century ago that the organization of body pattern might be controlled by socalled morphogen gradients. Only recently has it been possible to demonstrate that secreted proteins of the Transforming Growth Factor β (TGFβ), Wnt and Hedgehog families specify positional information by this mechanism. Drosophila Dpp is a member of the TGFβ superfamily and was the first secreted protein for which a morphogen function has been clearly demonstrated. Over the past ten years we have characterized the Dpp signaling pathway in detail, in collaboration with the group of Konrad Basler in Zurich.
Our studies provide the molecular framework for a mechanism by which the extracellular Dpp morphogen establishes a finely tuned, graded read-out of a transcriptional repressor complex including Smad proteins and the zinc-finger protein Schnurri. Targets of this repressor complex include transcriptional regulators as well as secreted proteins involved in morphogen transport. Other morphogens, which pattern the nervous system or the limb fields in higher vertebrates, might use similar mechanisms. Our current efforts are devoted to a systems biology approach and are done in the framework of the WingX project of the Swiss initiative in Systems Biology.
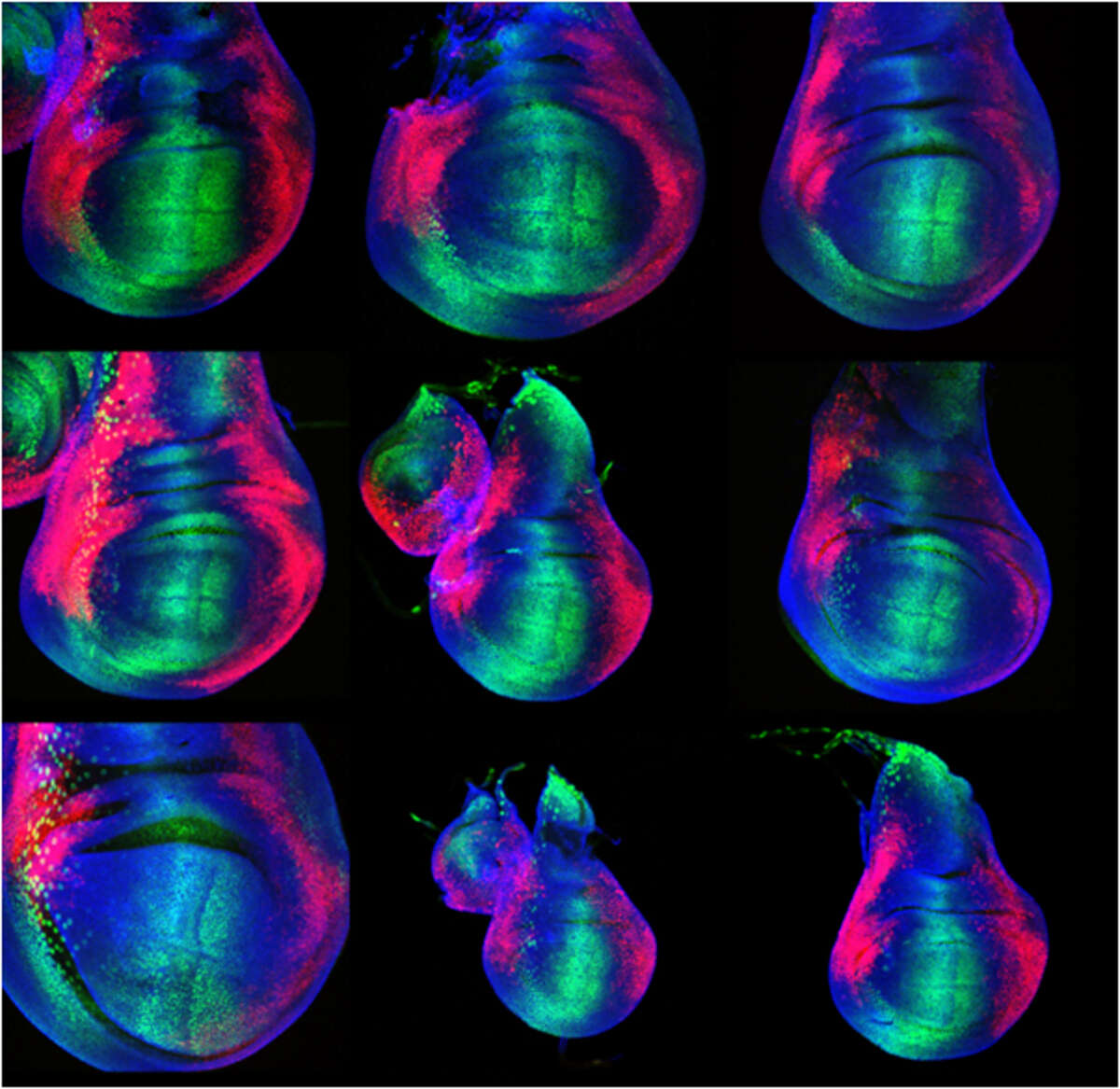
The experiments we concentrate on involve genome-wide target gene identification, real-time analysis of morphogen gradient readout, and computer modelling to better understand the dynamics of the Dpp morphogen system. Just recently, we have identified a novel feedback regulator of the Dpp system which controls the spreading of the Dpp molecule and might be involved in the adaptation of the morphogen gradient to tissue size. Our studies will eventually lead to a comprehensive understanding of morphogen function in tissue growth and patterning, a key issue in modern developmental biology (Figure 1).
Cell rearrangement in organ formation
To gain insight into how signaling pathways control more complex cellular decisions during the process of organ morphogenesis, we investigate the formation of the Drosophila tracheal system, an epithelial branched network similar to the lung, the kidney or the vasculature. Tracheal development serves as a paradigm to understand how epithelial cell sheets can be transformed by cell signaling and cell-cell or cell-matrix interactions into complex three dimensional networks, a process generally referred to as branching morphogenesis. Our approach has been to identify genes involved in the process by genetic analysis, and the characterization of relevant gene products by in vivo and in vitro analysis. In addition, we have devoted major efforts to characterize branching morphogenesis at the cellular level, using avant-garde, live imaging technology.
Over the past decade, these studies have provided a framework for understanding complex processes involved in the architectural design of developing organs, including the control and integration of cell migration and cell rearrangement via cell-cell signaling and extracellular matrix components (Figure 2).
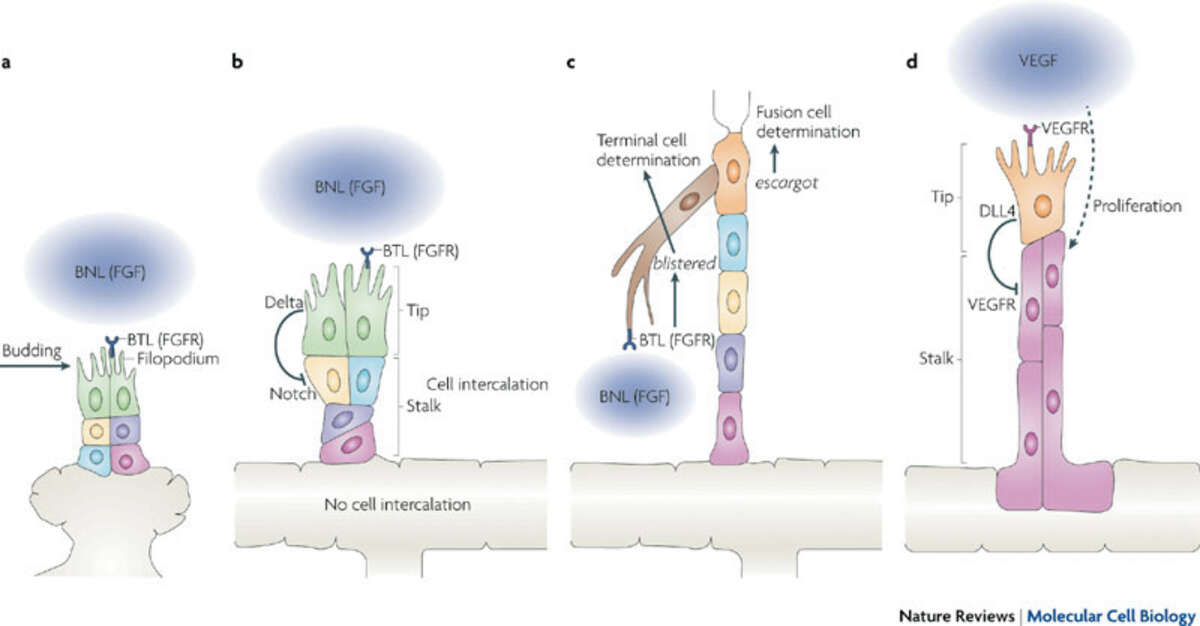
Fig. 2: Drosophila melanogaster trachea and vertebrate vasculature branching. Branchless (BNL), a fibroblast growth factor (FGF), acts at the top of the hierarchy of cellular events that orchestrate tracheal branching in Drosophila melanogaster (a to c). During vertebrate angiogenesis, vascular endothelial growth factor (VEGF) signalling determines the formation of angiogenic sprouts and controls tip cell and stalk cell identity through Delta Notch signalling. Taken from Affolter et al. (2009) Nat Rev Mol Cell Biol 10, 831-42.
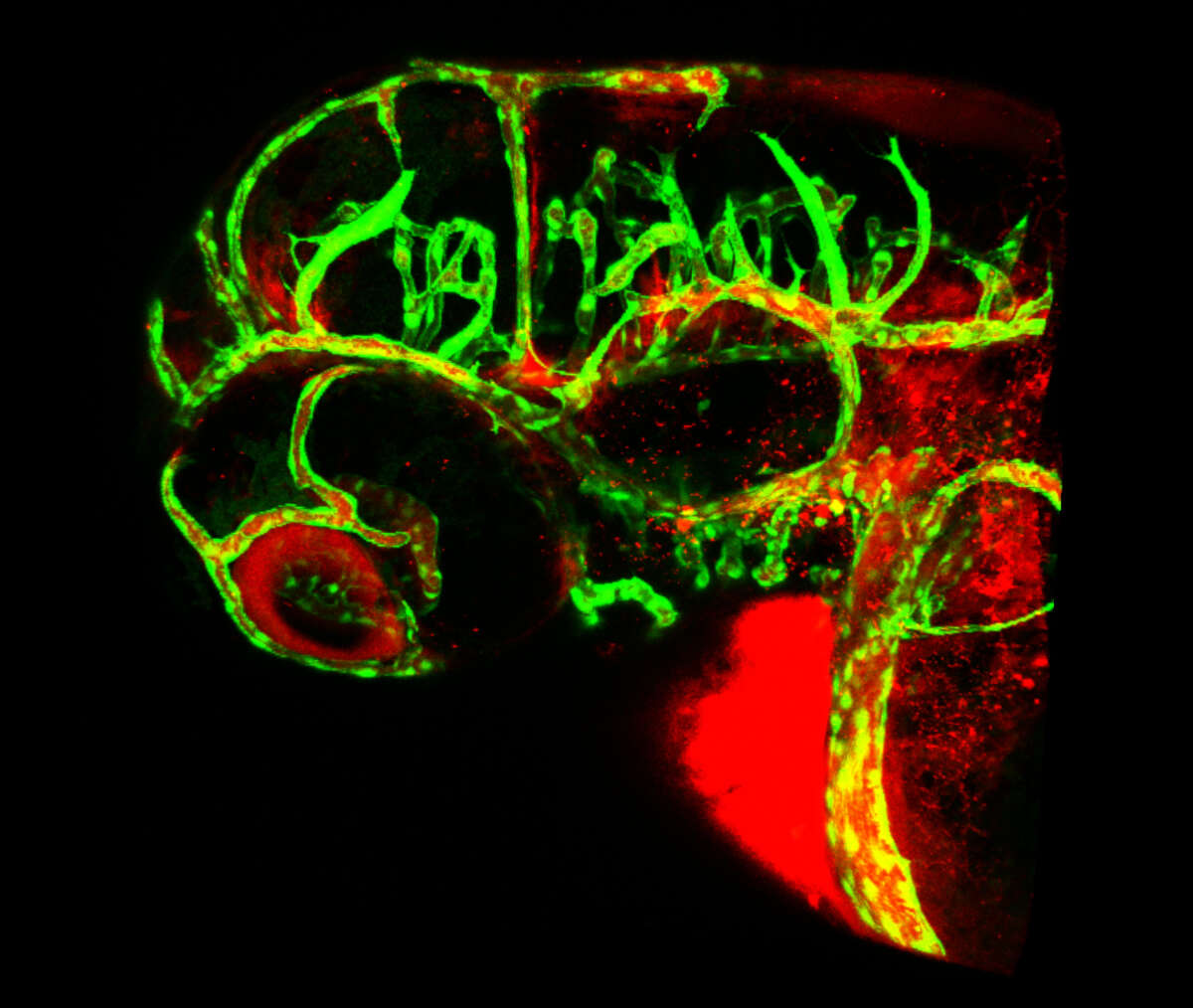
Studies on the development of blood vessels in higher organisms suggest strong parallels between tracheal development in insects and tube formation in the growing vasculature (see Figure 2). Interested by this possible developmental similarity, we have initiated studies aimed at a better understanding of blood vessel development in zebrafish, one of the most promising animal systems in the study of angiogenesis available at the moment. We have indeed found that our approach to studying cell rearrangement during tracheal development provides a novel insight into how cells behave during angiogenesis when applied to zebrafish. We have recently proposed a novel model for the architecture of the first vessels formed via angiogenesis, a model which is strikingly different to the one previously described. Our studies re-define the cellular routines involved in angiogenesis, and provide the basis for all future studies in the zebrafish regarding angiogenesis. We have now strengthened our efforts to study angiogenesis using live imaging combined with novel transgenic lines and strategies Figure 3). Particular emphasis is devoted to the study of blood vessel fusion, a process that has not been studied in the past in vivo at the cellular level.
Interested by this possible developmental similarity, we have initiated studies aimed at a better understanding of blood vessel development in zebrafish, one of the most promising animal systems in the study of angiogenesis available at the moment. We have indeed found that our approach to studying cell rearrangement during tracheal development provides a novel insight into how cells behave during angiogenesis when applied to zebrafish. We have recently proposed a novel model for the architecture of the first vessels formed via angiogenesis, a model which is strikingly different to the one previously described. Our studies re-define the cellular routines involved in angiogenesis, and provide the basis for all future studies in the zebrafish regarding angiogenesis. We have now strengthened our efforts to study angiogenesis using live imaging combined with novel transgenic lines and strategies (Figure 3). Particular emphasis is devoted to the study of blood vessel fusion, a process that has not been studied in the past in vivo at the cellular level.
Interested by this possible developmental similarity, we have initiated studies aimed at a better understanding of blood vessel development in zebrafish, one of the most promising animal systems in the study of angiogenesis available at the moment. We have indeed found that our approach to studying cell rearrangement during tracheal development provides a novel insight into how cells behave during angiogenesis when applied to zebrafish. We have recently proposed a novel model for the architecture of the first vessels formed via angiogenesis, a model which is strikingly different to the one previously described. Our studies re-define the cellular routines involved in angiogenesis, and provide the basis for all future studies in the zebrafish regarding angiogenesis. We have now strengthened our efforts to study angiogenesis using live imaging combined with novel transgenic lines and strategies (Figure 3). Particular emphasis is devoted to the study of blood vessel fusion, a process that has not been studied in the past in vivo at the cellular level.