Main Content
Principles of intracellular organization
Asymmetry is an inherent property of most cells. Proteins and mRNA have to be distributed at specific cellular locales to perform their assigned function or to be translated in a spatially and temporally regulated manner. Although the localization of mRNAs is restricted to the cytoplasmic face of intracellular organelles or the plasma membrane, proteins and lipids have to be localized to these organelles to provide a platform on which mRNAs and/or proteins can be recruited and form a domain or a small compartment. In general this compartmentalization is achieved by intracellular transport through exocytic (secretory pathway) and endocytic avenues. Communication between different organelles is maintained in large parts by transport vesicles and direct contact sites. While the transport vesicles transport proteins and lipids, the contact sites are places for ion and lipid exchange. These different kinds of communications not only are essential for cell asymmetry establishment and maintenance they further allow to mount a rapid cellular response in case of stress.
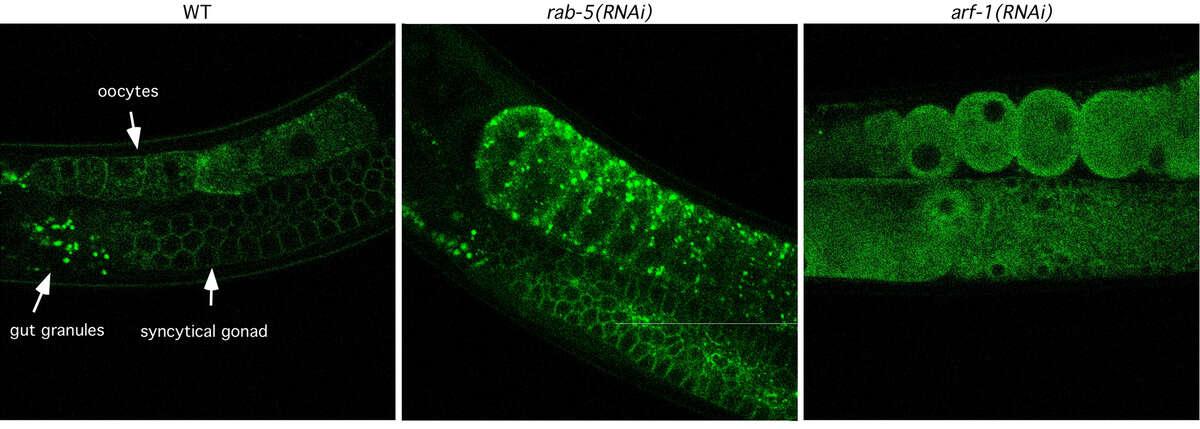
Fig. 1: The architecture of the Golgi is disturbed upon knockdown of the small GTPases RAB-5 and ARF-1. Worms expressing the Golgi marker UGTP-1::GFP (green) under the pie-1 promoter, which drives expression in the gonad and in early embryos were subjected to RNAi by feeding. The distribution of UGTP-1::GFP was analyzed by confocal microscopy. The Golgi morphology was greatly altered upon RNAi against RAB-5 and ARF-1. In particular, arf-1(RNAi) let to a dispersal of Golgi structures.
Our research interests center around questions like: how intracellular traffic regulates cellular asymmetry and stress response. How do RNA localization, translation and metabolism contribute to asymmetry and defense against stressors. To answer these questions we take advantage of different model systems such as yeast, C. elegans and tissue culture to enable us to study these problems from the single cell to organ and animal level.
The regulation transport along the secretory pathway
In order to understand how microdomains or compartments are formed on different organelles and the plasma membrane, we have to understand how proteins reach their specific compartments. Therefore, we study on one hand the formation of transport containers at different levels of the secretors pathway. Since the formation of transport containers is mostly dependent on the small GTPase Arf1, we study its regulation through ArfGEFs and ArfGAPs (Ackema et al., 2013, 2014, Estrada et al., 2014, Spang et al 2010, Spang 2009, 2013). Activated Arf1 will interact with adaptor complexes and coat components that will on one hand recruit the cargo that needs to be transported and on the other hand deform the donor membrane to allow the generation of a transport vesicle. We have identified a novel adaptor complex, exomer, which only transport a subset of proteins from the trans-Golgi network (TGN) to the plasma membrane (Trautwein et al., 2006, Zanolari et al., 2010, Rockenbauch et al., 2012, Spang 2015, Huranova et al., in press). Yet the cargoes are all exquisitely regulated in terms polarized localization through the cell-cycle and upon stress response (Zanolari et al., 2010, Ritz et al., 2014). One of the cargoes, Pin2, contains a prion-like domain, which serves as a TGN retention signal, in particular under stress conditions (Ritz et al., 2014). The prion-like aggregates may also serve to sequester other cargo proteins, hence providing a novel mechanism for compartmentalization and domain formation.
The regulation of early-to-late endosomal transport
However not only export to the plasma membrane is essential for maintaining cell polarity and asymmetry, the endocytic route plays an equally important role. We have identified a factor, SAND-1/Mon1, which acts as a switch in early to late endosome maturation by on one hand displacing the GEF for the early endosomal GTPase RAB-5, RABX-5, from early endosome and by actively recruiting the late endosomal GTPase RAB-7 onto the maturing endosome (Poteryaev and Spang 2005, Poteryaev et al., 2007, 2010). With these results, we demonstrated the existence of endosome maturation. But then the next question is how is maturation regulated? Rab conversion from RAB-5 to RAB-7 positive endosomes is an important but not the only step along the maturation process. We are studying currently how recycling and sorting, pH and lipid changes, interactions with the cytoskeleton, incoming cargo from the TGN and signaling pathways influence endosome maturation and how these different processes are coordinated. Recently, we demonstrated that the SM subunits of the HOPS and CORVET tethering complexes (Solinger and Spang, 2013) are important to control the flow through the endocytic pathway (Solinger and Spang, 2014). This project led us also to the discovery of a novel set of tethering complexes in the endocytic pathway.
The regulation of mRNA metabolism and transport
This research direction was inspired by our finding that the poly A binding protein, Pab1, associates with Arf1 and COPI vesicles in an mRNA-dependent manner and that Arf1 is required for ASH1 mRNA localization to the bud tip of yeast cells (Trautwein et al., 2004). The subsequent analysis allowed us to identify the first distal pole-localized mRNA in yeast (Kilchert and Spang, 2011). We performed screens to identify mRNAs that are restricted to certain sites in the cell, and we are currently investigating the mechanism of the localization of a subset of mRNAs. Moreover, we realized that arf1 mutant caused the formation of processing bodies (P-bodies), which are the main mRNA degradation platform in yeast (Kilchert et al., 2010). Interestingly, the P-bodies formed in arf-1 and other secretory pathway mutants were morphologically distinct from the ones formed under glucose starvation and required signaling through Ca2+/calmodulin. This led to the hypothesis that P-bodies may sequester a distinct set of mRNAs depending on the stressor. To test this hypothesis, we established a protocol that allowed us to purify P-bodies and to determine the RNA content through RNAseq. Likewise, we have established ribosome profiling to be able to get a systems view on how the cell regulated its proteome through mRNA degradation and translation under various conditions. This latter part was based on previous findings that polysome-associated proteins negatively control P-body formation under normal growth conditions (Weidner et al., 2014).