Main Content
Selective transport control in biomimetic and cellular systems
Karyopherin-centric control of nuclear pores (SNF/SNI)
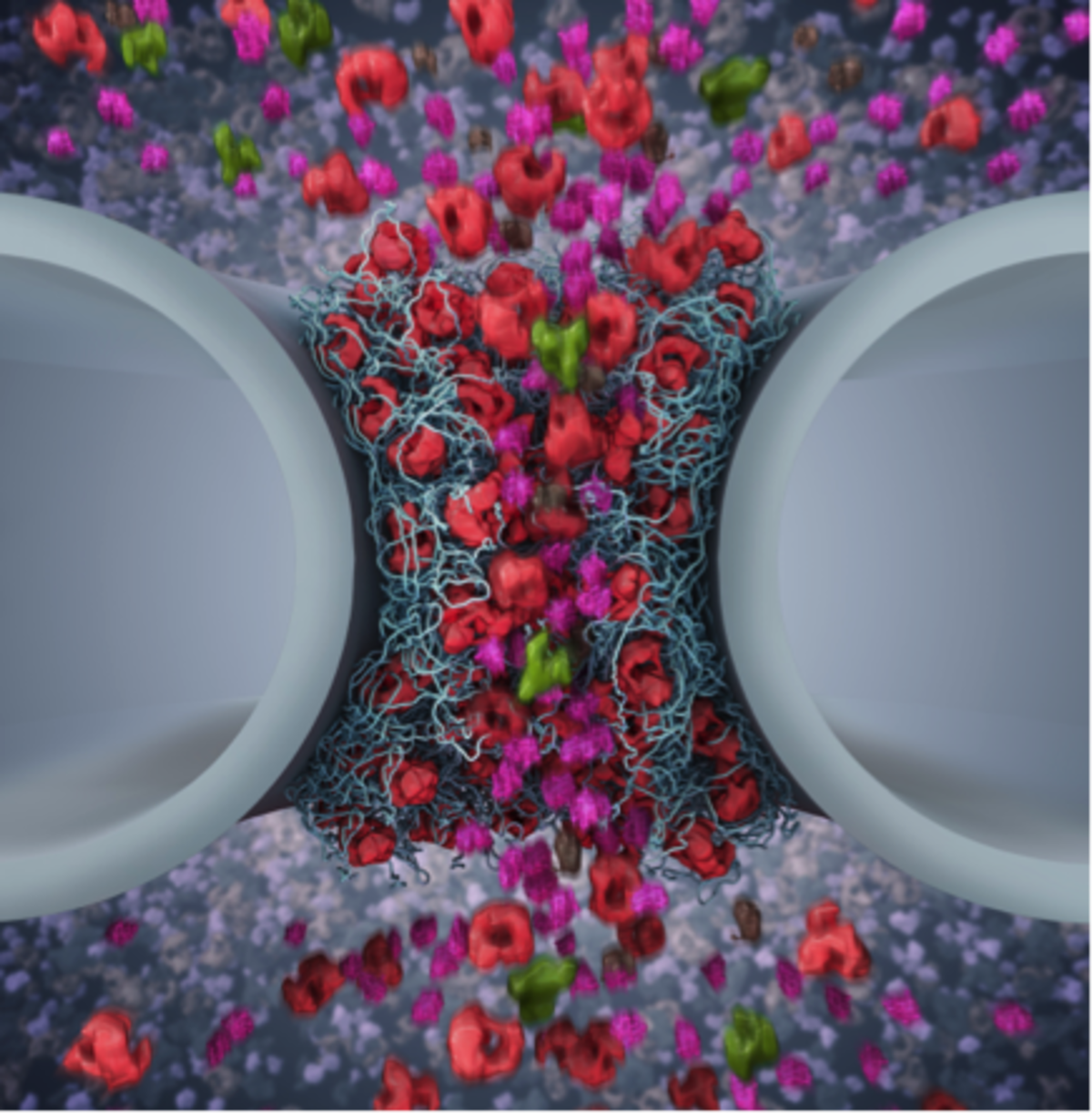
Nuclear pore complexes (NPCs) are 50 nm-diameter aqueous channels that function as the main transport hubs between the cytoplasm and nucleus in eukaryotic cells. Exclusive access is orchestrated by nuclear transport receptors (NTRs) that interact with intrinsically disordered proteins known as FG Nups that otherwise generate a permeability barrier within the NPC. Our interest is to understand how multivalent NTR-FG Nup binding impacts on FG Nup barrier function and transport kinetics at the (i) biophysical level, (ii) within individual NPCs, and (iii) in cells. Our findings thus far indicate that a classical NTR known as Kapβ1 acts as a bona fide constituent of the FG Nup barrier and plays a role in modulating selective transport control in NPCs.
Biomimetic systems for selective transport control (NCCR Molecular Systems Engineering)
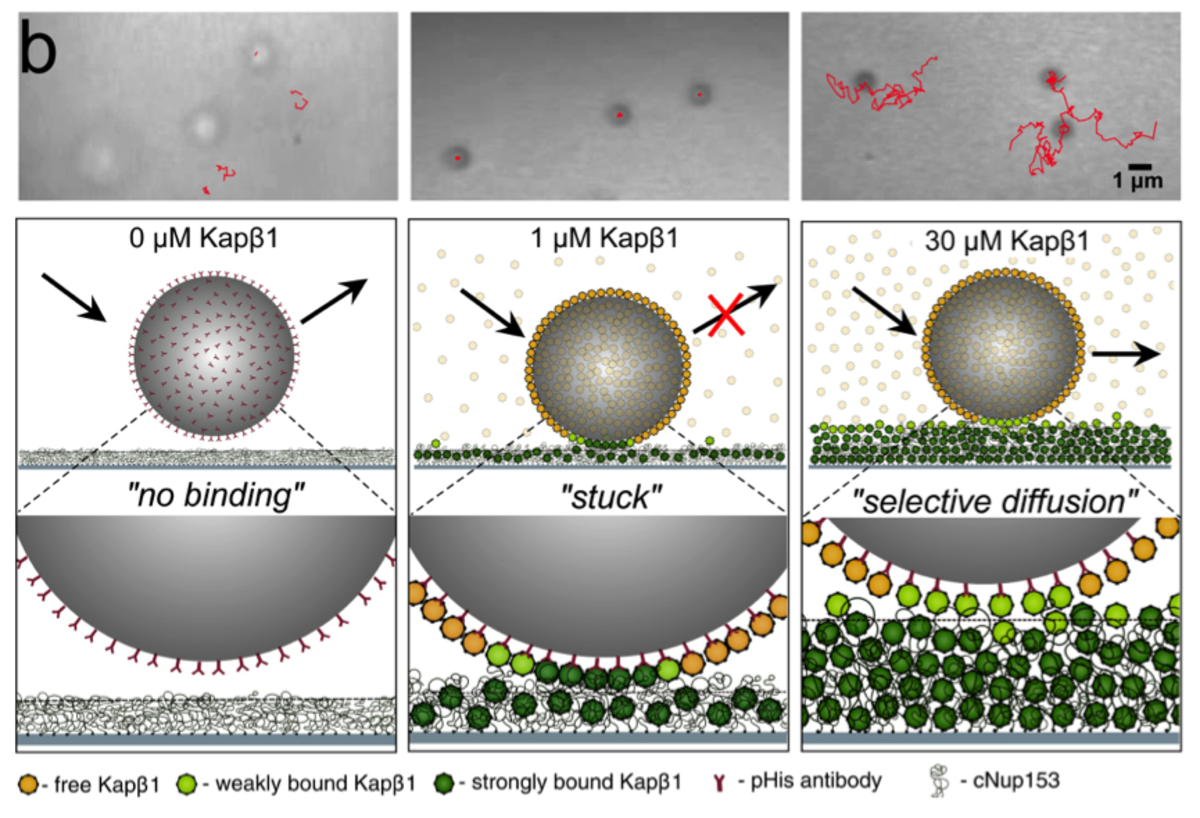
Nucleocytoplasmic transport is regulated by soluble nuclear transport receptors (NTRs) that exclusively enter NPCs to accumulate selective cargoes in the nucleus against concentration gradients. Here, we want to exploit this phenomenon as a means of implementing selective transport control in biomimetic systems. To do so, we are engineering molecular adaptors that can mediate between biological and synthetic systems. This includes constructing NPC-inspired biomimetic nanopores, as well as implementing two-dimensional transport control using the so-called “dirty velcro effect”.
The mechanobiological hallmarks of living tissue (NanoTera)
Cells adapt to their microenvironments via vast mechanosensory networks that have a reciprocal effect on cellular stiffness and mechanophenotype.
In epithelia, these mechanosignals are transmitted from intercellular junctions and basal contacts formed on basement membranes (BMs) into nuclei. We are investigating how mechanosignaling regulates cell polarity, cytoskeletal organization, and inter-cellular communication. Interestingly, our findings reveal that epithelial cells on native BMs exhibit several key features of living tissue that are suppressed in reconstituted basement membrane (i.e., Matrigel).
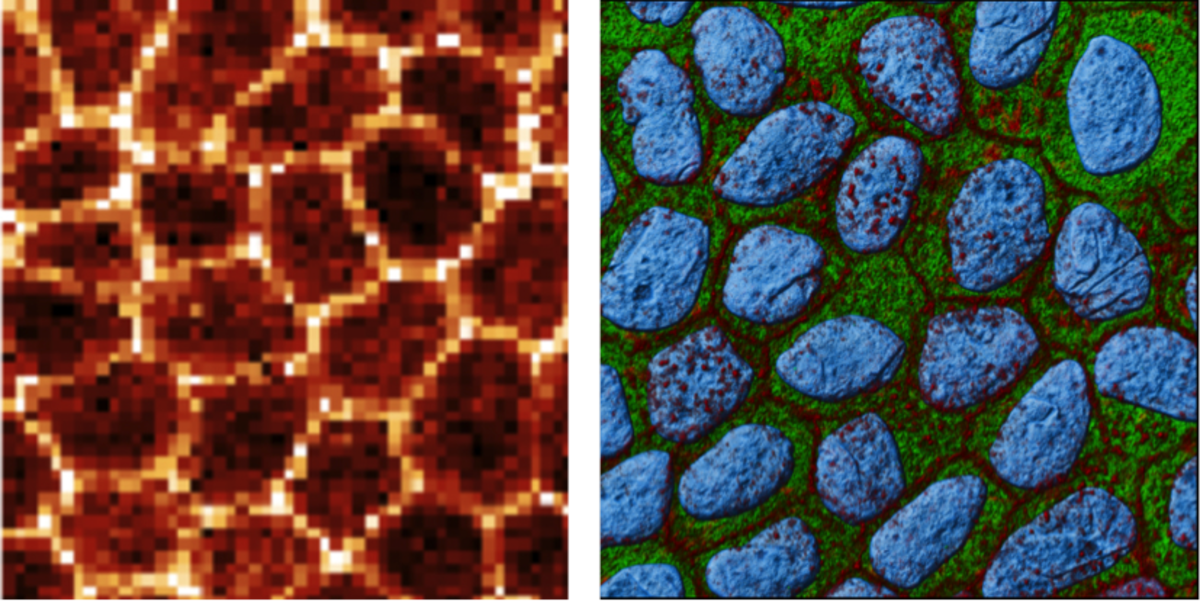
Fig. 3: ARTIDIS stiffness (left) and immunofluorescence (right) images show epithelial layer formation when cultured on native basement membrane (green) that reveals actin (red) cytoarchitecture typical for epithelial sheets in vivo. The cell nucleus is depicted in blue. Image: Philipp Oertle (Bioz, Basel) and Vasily Gurchenkov (institute Curie, Paris).
ARTIDIS and nanomechanical tissue diagnostics (KTI)
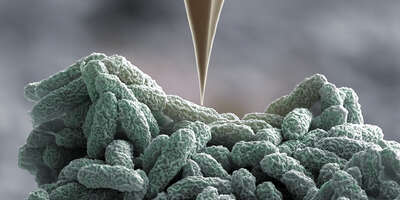
We have built an atomic force microscope (AFM)-based apparatus (i.e., ARTIDIS “Automated and Reliable Tissue Diagnostics”) to measure cellular stiffness with unsurpassed sub-cellular spatial resolution and high force sensitivity within living tissue.
By examining unadulterated human breast cancer biopsies, we have uncovered how the “softness” of cancer cells is associated with hypoxia and is fundamentally coupled to their propensity to invade and metastasize. This has resulted in a spin off ARTIDIS® that is commercializing the use of nanomechanical tissue diagnosis for clinical applications.


Fig. 4: Cover image depicting the principle of ARTIDIS, which uses a sharp nanometer-sized AFM tip to probe the stiffness of an invasive cancer cell that is seen protruding above a malignant matrix-embedded cluster (Plodinec et al, Nature Nanotechnology 2012). Image: Eva Bieler, Marija Plodinec and Roderick Lim. Artwork: Martin Oeggerli/Micronaut 2012. Cover Design: Alex Wing.