Main Content
Research Projects
Our team is comprised of people with different training: Some team members are trained in physics or mathematics, some are trained in molecular biology, and others are trained in interdisciplinary sciences or chemistry or engineering.
Our research group focuses on understanding physical and biological mechanisms of multicellular bacterial behaviors. As model systems, we use bacterial biofilm formation and swarming. By combining custom-built microscopy, transcriptomics, molecular biology techniques, statistics, machine learning, and mathematical modeling, we study how bacteria form complex multicellular communities, how these communities obtain emergent functions, and how these communities affect bacterial ecology.
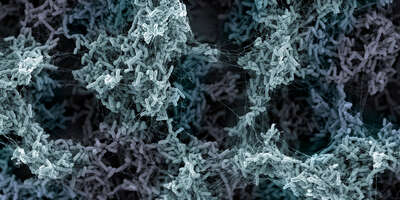
Biofilm formed by three different Vibrio cholerae strains, each expressing a different fluorescent protein.
Biofilm dynamics: From growth to dispersal
Which cell-cell interactions determine biofilm architecture? And how do cells decide when they should disperse from biofilms? To understand how biofilms emerge from the interactions of all cells, we developed 3D image analysis techniques which allow us to track all individual cells in biofilms, revealing beautiful internal cellular arrangements, and the different stages of biofilm growth. [Drescher, et al. 2016; Hartmann, et al. 2019; Hartmann, et al. 2021; Jelli, Ohmura, Netter, et al. 2023; Ohmura, et al. 2024]. We have made these image analysis tools available in the form of a software tool, BiofilmQ [Hartmann, et al. 2021] and a tool for bacterial cell segmentation in 3D images, StarDist OPP [Jelli, Ohmura, Netter, et al. 2023]. We sometimes also build custom-made microscopes, e.g. for confocal or light sheet microscopy [Yordanov, Neuhaus, et al. 2021].
Mechanical interactions dominate short-range interactions: These single-cell level data during biofilm development can be used to constrain hypotheses about key cell-cell interaction mechanisms that take place during biofilm growth. We recently found that mechanical interactions are dominant during early biofilm development [Hartmann, et al. 2019]. Similarly, mechanical interactions are important during swarm development [Jeckel, et al. 2019a; Jeckel, et al. 2019b]. We also discovered that biofilms of different bacterial species share a universal biofilm architecture, which is determined by physical interactions between the cells [Jeckel, Diaz-Pascual, Skinner, Song, et al. 2022]. Interestingly, living multicellular systems display a different architecture from non-living systems with many constituent components [Skinner, et al. 2023]. Also during exposure to high flow rates, mechanical interactions shape biofilm growth, even if high flow rates modulate the mechanical interaction strength [Pearce, et al. 2019; Ohmura, et al. 2024]. Biofilm interactions with flow are technologically highly relevant, and we have characterized several interesting phenomena for biofilm behaviors in flow, including the dynamics of streamer formation [Drescher, et al. 2013; Kim, et al. 2014].
Non-mechanical interactions dominate long-range interactions: We developed techniques for measuring spatial transcriptomes in bacterial communities - for biofilms [Diaz-Pascual, et al. 2021] and for swarms [Jeckel, Nosho, et al. 2023]. These spatial/spatiotemporal transcriptome techniques revealed many new cell-cell interactions that take place on longer length scales than mechanical interactions.
Biofilm dispersal: Cells often do not stay in a biofilm forever. Yet it is unclear how cells reach a decision for when they should decide to disperse, and how they disperse. For Vibrio cholerae, we recently discovered that cells monitor a self-secreted quorum sensing signal, and the local nutrient concentration, to reach robust decisions about dispersal as a collective [Singh, et al. 2017].
Biofilms in infections, ecology, and evolution - why do bacteria form biofilms?
Infections: We recently discovered that biofilms can form around human immune cells, to kill the immune cells [Vidakovic, et al. 2023]. Biofilm formation around immune cells inverts the typical predator-prey interaction between bacteria and immune cells. We also discovered that bacterial-fungal mixed species biofilms on teeth strongly promote early childhood caries [Ren, Jeckel, et al. 2022].
Antibiotics: Bacteria that are bound in biofilms are highly resistant against antibiotics and other chemical insults of the environment, which is a clear evolutionary advantage of forming biofilms. Remarkably, the mechanisms underlying the biofilm-antibiotic interaction is poorly understood, and we are investigating unicellular and multicellular responses to antibiotics in biofilms [Diaz-Pascual et al. 2019].
Phage-biofilm interactions: Apart from providing protection against toxins, evolutionary advantages to biofilm formation are vague. However, we recently found the mechanisms underlying the most important selective advantage of making a biofilm: predation avoidance by bacteriophages. [Vidakovic, et al. 2018; Simmons, et al. 2018; Simmons et al. 2019]
Social behaviors in biofilms: We also discovered another reason for why bacteria may want to form biofilms: physical aspects of the biofilm life style strongly favor the evolution of simple social behaviors, such as the production of shared resources or "public goods" [Drescher, et al. 2014; Nadell, et al. 2013]. In addition, we are investigation social interactions in spatially structured biofilm communities [Dragos, et al. 2018; Nadell, et al. 2016].
Physics of bacterial collective behaviors
What can we learn about collective bacterial behaviors from physics? Many aspects of bacterial interactions are inherently physical. Some examples: During biofilm growth, cells push and pull on each other, while being embedded in an elastic matrix. Understanding the molecular transport of nutrients and metabolites through the biofilm also relies on physics. Before bacteria form biofilms, their swimming motility creates fluid flows that lead to physical interactions with surfaces and other bacteria.
[Ohmura, et al. 2024; Jeckel, Nosho, et al. 2023; Hartmann, et al. 2019; Jeckel, et al. 2019; Drescher, et al. 2011; Wensink, et al. 2012; Dunkel, et al. 2014]