Many bacteria adopt a fascinating defense strategy by forming communities on surfaces, known as biofilms. We encounter such biofilms in our daily lives, for example, as dental plaque in the mouth, slimy films on stones in water or even as part of our intestinal flora. Bacterial biofilms are intrinsically tolerant to antibiotics and can pose a significant threat in clinical settings when they colonize implants, catheters, or surgical instruments. This colonization enables pathogens to infiltrate our body and trigger infections that are difficult to combat by the immune system and with antibiotics.
Previously, it was assumed that bacteria form biofilms to defend and protect themselves. The research team led by Prof. Knut Drescher at the Biozentrum, University of Basel, has now demonstrated, in their recently published “Cell” study, that bacteria form biofilms on the surface of immune cells. This previously unknown type of community differs from already known bacterial biofilms not only in its structure, but also in its function: instead of serving a protective purpose, this biofilm is an aggressive trait.
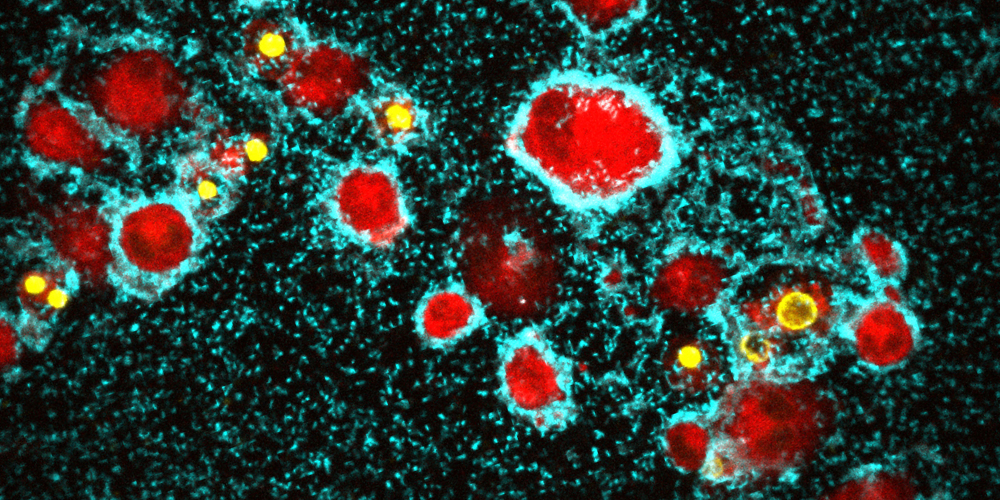
Biofilm on immune cells: meshwork rather than typical slimy matrix
Drescher’s team has discovered this novel type of biofilm in the cholera-causing pathogen Vibrio cholerae. This bacterium colonizes various immune cells in the human host. To better understand biofilm formation on immune cells, the researchers focused on a certain type of phagocytic cells, so-called macrophages.
“Bacteria that accidently encounter a macrophage attach to the cell’s surface using a kind of a ‘feeler’,” explains first author Lucia Vidakovic. “Subsequently, the bacteria start to divide and intertwine their feeler-like appendages.” The structure of the extracellular matrix of this type of biofilm is thus fundamentally different from previously known ones, in which bacteria are typically embedded within a slimy matrix consisting of sugars and proteins.
Biofilms on immune cells are an aggressive instead of defensive strategy
Over time, the biofilms produced by the cholera pathogen completely encase macrophages, leading to cell death. “The bacterial community actively attacks and kills the immune cells. However, we initially didn’t understand the exact mechanism,” says Vidakovic. “To solve this puzzle, we meticulously investigated all 14 known toxins produced by the cholera pathogen and could finally identify the hemolysin as the culprit.” This toxin forms pores in the protective membrane of the immune cells, thus killing them.
Miniature intestine as a model system
Cholera is a life-threatening infectious disease that causes severe diarrhea. As humans are the only host of the cholera-pathogen, the scientists established a human intestinal organoid model. Using this model, they could demonstrate that Vibrio cholerae is able to form lethal biofilms on macrophages after colonizing and disrupting the human intestinal barrier.
“This novel strategy of attack, employed by the bacteria, can significantly affect the progression of the cholera infection,” adds Knut Drescher. "In a next step, we aim to explore whether other pathogens also form such aggressive biofilms. Deciphering the strategies of bacterial pathogens is crucial for the development of new approaches to fight them."
NCCR AntiResist
The study is part of the National Centre of Competence in Research (NCCR) "AntiResist" and was conducted in collaboration with the École Polytechnique Fédérale de Lausanne. The aim of this research network is the discovery of new antibiotics and the development of novel strategies to combat antibiotic-resistant pathogens.
Original publication:
Lucia Vidakovic, Sofya Mikhaleva, Hannah Jeckel, Valerya Nisnevich, Kerstin Strenger, Konstantin Neuhaus, Keerthana Raveendran, Noa Bossel Ben-Moshe, Marina Aznaourova, Kazuki Nosho, Antje Drescher, Bernd Schmeck, Leon N. Schulte, Alexandre Persat, Roi Avraham, Knut Drescher. Biofilm formation on human immune cells is a multicellular predation strategy of Vibrio cholerae. Cell, published online 8 June 2023.
Contact: Biozentrum Communications