Main Content
Stem cells and their niche in the adult mammalian brain
Neural stem cells continuously generate new neurons in restricted areas of the adult mammalian brain. Adult stem cells reside in specialized niches that support their lifelong capacity for self-renewal and differentiation. Intriguingly, adult neural stem cells exhibit features of glial cells, raising the possibility that glial cells elsewhere in the brain may have latent stem cell potential. The ventricular-subventricular zone (V-SVZ), adjacent to the lateral ventricles, is the largest germinal niche in the adult brain and continuously gives rise to neurons that migrate to the olfactory bulb, as well as small numbers of glia (Chaker et al, 2016). We are investigating the intrinsic and extrinsic (niche) signals that regulate adult V-SVZ neural stem cells under homeostasis and during regeneration.
Intrinsic regulation of adult neural stem cells and their lineage
A key limitation in the adult neural stem cell field has been the identification of markers that allow the in vivo identification and isolation of adult neural stem cells. We have recently developed a simple strategy that allows the purification of both quiescent (dormant) and activated (dividing) adult neural stem cells as well as their progeny using fluorescence activated cell sorting (FACS) (Pastrana et al 2009, Codega et al 2014). Quiescent and activated stem cells isolated directly from their in vivo niche exhibit dramatically different cell cycle, molecular, and functional properties and have unique transcriptional signatures. This FACS purification strategy provides a powerful approach to investigate the biology of adult neural stem cells. In ongoing work we are defining the heterogeneity and potential of purified populations, as well as the gene regulatory networks underlying adult neural stem cell quiescence and activation.
Niche regulation of adult neural stem cells
V-SVZ stem cells span different compartments of the stem cell niche. They contact both the cerebrospinal fluid (CSF), which flows through the ventricles, and the vasculature. We are using a combination of novel anatomical and in vitro approaches to elucidate the diverse niche components that mediate adult neural stem cell behavior.
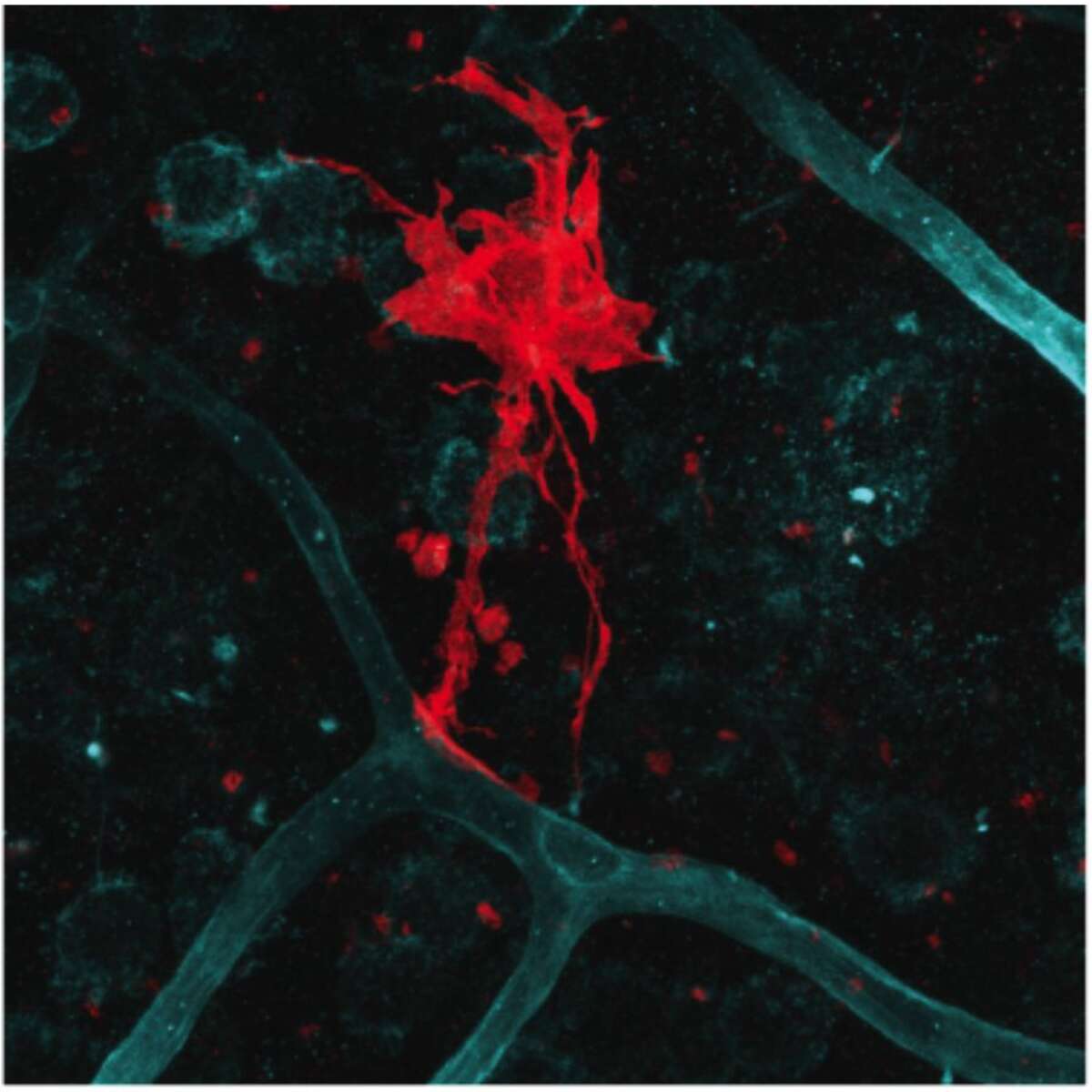
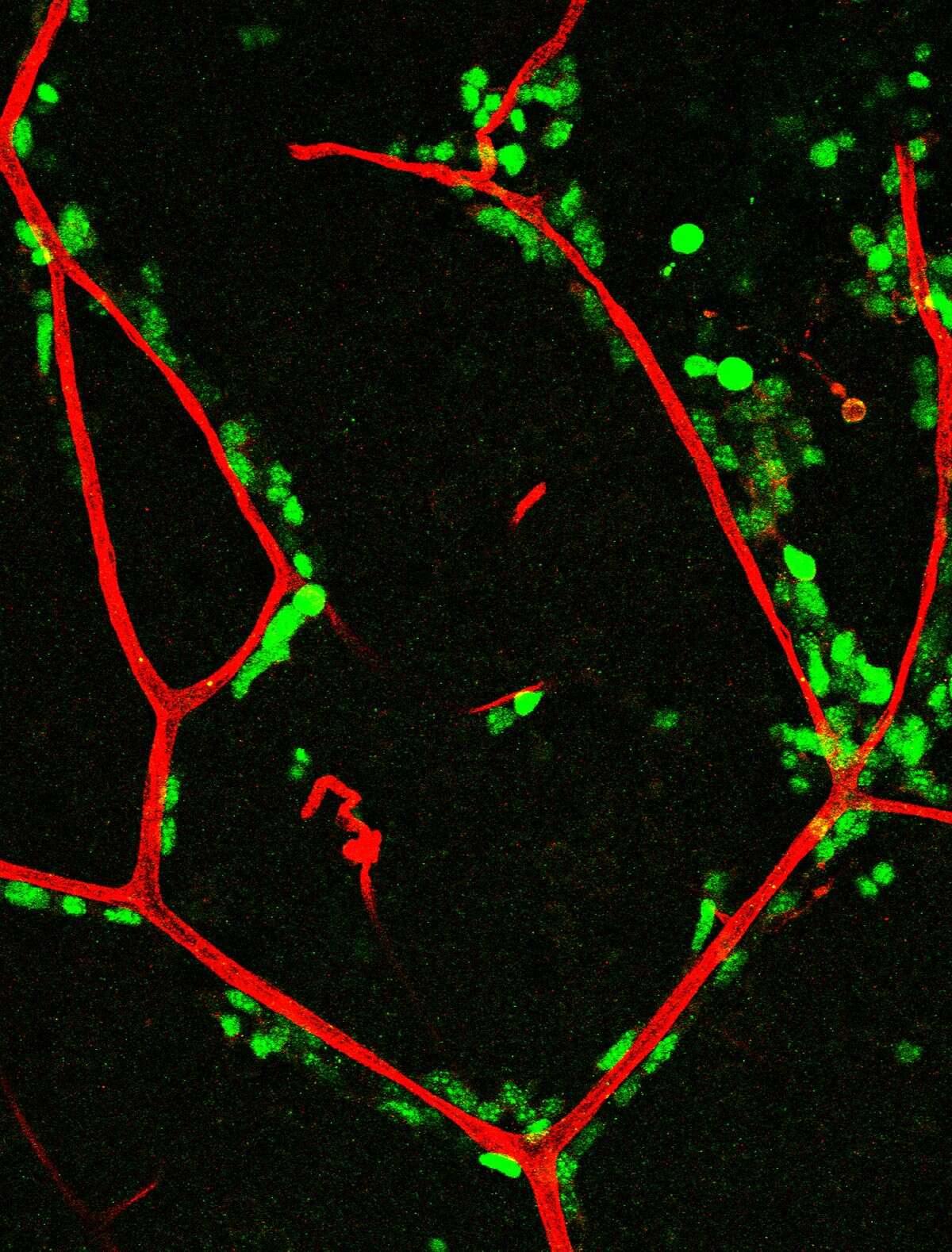
Vascular niche: The vasculature is an important component of the V-SVZ neural stem cell niche, and has unique properties (Tavazoie et al, 2008). Stem cells directly contact blood vessels at specialized sites that lack astrocyte endfeet. An open question is whether vascular cells in neurogenic areas are intrinsically different from those elsewhere in the brain. We have optimized a rapid FACS purification strategy to simultaneously isolate primary endothelial cells and pericytes from brain micro-regions of non-transgenic mice (Crouch et al, 2015). This purification strategy provides a platform to define the functional and molecular contribution of vascular cells to stem cell niches and other brain regions under different states. We are defining endothelial and pericyte-derived factors that influence V-SVZ cells.
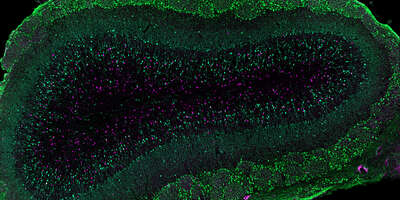
Choroid plexus/Cerebrospinal fluid niche: Adult neural stem cells contact the lateral ventricle via a thin process, and are continuously bathed by the cerebrospinal fluid (CSF). However little is known about the effect of the CSF compartment on adult neural stem cell function. The CSF is produced by the choroid plexus, which floats in the brain ventricles. Although the lateral ventricle choroid plexus (LVCP) is in close proximity to the V-SVZ, it has largely been ignored. We have found that in addition to homeostatic functions, the LVCP is a key component of the adult V-SVZ NSC niche (Silva-Vargas et al, 2016). LVCP secreted factors promote colony formation and proliferation of purified quiescent and activated V-SVZ NSCs, as well as of transit amplifying cells. Moreover, the functional effect of the LVCP secretome changes during aging. In ongoing work we are investigating how the LVCP dynamically responds to different physiological states and affects adult neural stem cell behavior.