Cholesterol-lowering agents, antihypertensives or malaria medications, cancer drugs and antibiotics - many of our current drugs are based on natural products. Bacteria and fungi actually produce these chemical substances in order to fight competitors or to coordinate co-existence with partners in peaceful symbiosis. This ability of microbes is applied specifically for the biotechnological production of drugs and often inspires the chemical synthesis of drug-like compounds.
Blueprint determines product
In the microorganisms, molecular factories, the so-called polyketide synthases, yield a broad spectrum of potent products, the polyketides. These factories link individual chemical components to form long chains in a progression of individual steps, like on an assembly line. Each polyketide factories consists of different production sites, which determine the final product. Together with partners from the Johns Hopkins University in Baltimore, Prof. Timm Maier's group at the Biozentrum of the University of Basel has now elucidated how a loading station at the beginning of such an assembly line is embedded in the molecular factory.
Structure of factory coordinates processing steps
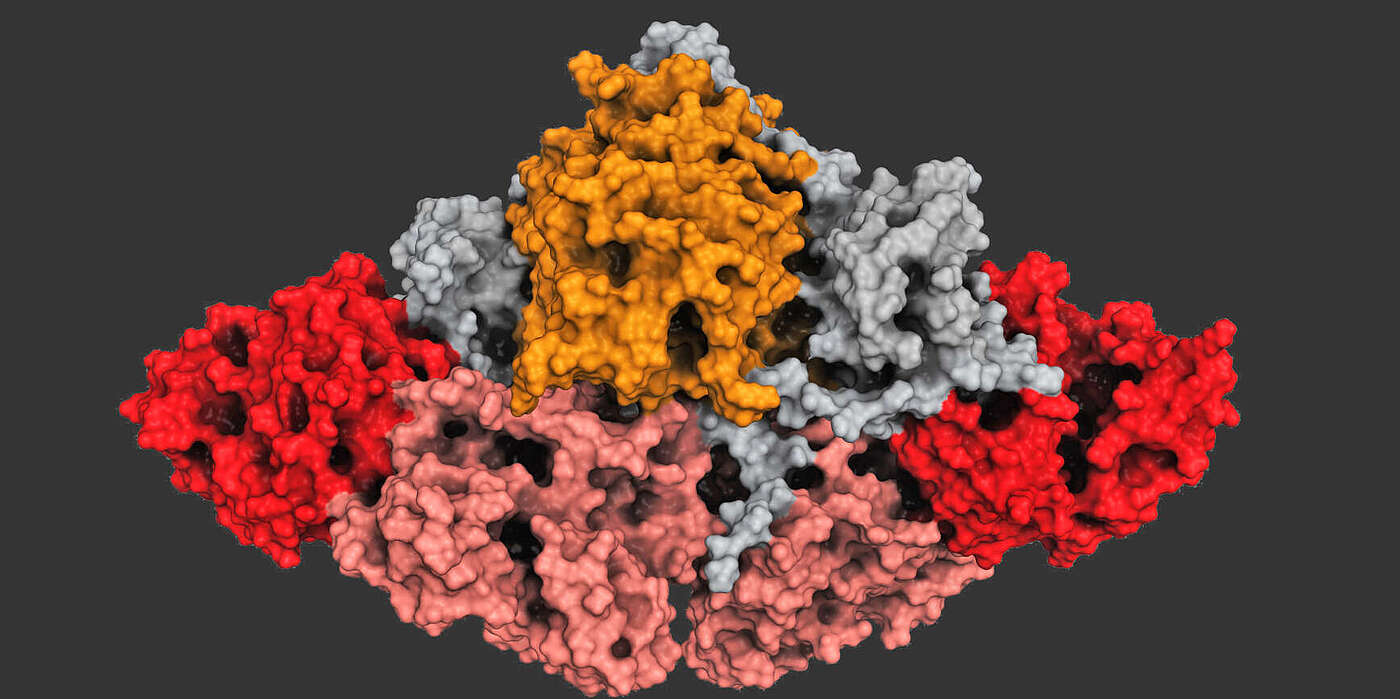
The polyketide synthase studied consists of two identical protein strands, each containing five enzymatic units and a carrier protein. The synthesis of a product begins with loading a short starter molecule at the loading station to a carrier protein. Subsequently, the other enzyme units attach new components to the starter molecule, stepwise elongating the chain until the product is finished. The crystal structure clearly shows that the loading station – contrary to earlier belief – is firmly integrated into the factory.
Using a chemical trap, the researchers were able to capture a snapshot of the factory at the end of the loading step and to structurally analyze it by electron microscopy. This revealed that the work of the two strands is coupled. "Loading on one strand influences the structure of the other," says Dominik Herbst, first author of the publication. "This results in the individual production steps in the factory process being coordinated."
Re-engineered factories to produce new drugs
Today, it is extremely complex and time-consuming to produce new bioactive agents based on polyketides. With their study, Maier and his team provide crucial information for the synthesis of improved or even novel drugs. "Once we understand how the individual stations of the polyketide assembly lines work together, we can reassemble and repurpose them," says Maier. This opens the door for the production of better or even yet unknown drugs.
Original article:
Dominik A. Herbst, Callie R. Huitt-Roehl, Roman P. Jakob, Jacob M. Kravetz, Philip A. Storm, Jamie R. Alley, Craig A. Townsend and Timm Maier. The structural organization of substrate loading in iterative polyketide synthases. Nature Chemical Biology, published online 2 April 2018
Contact: Communications, Katrin Bühler