Although cells are separated from the outside world by a membrane, they can still sense well what is happening around them. They capture hormones and nutrients such as sugar, iron or cholesterol and bring them packed in vesicles into the cell’s interior. Likewise, proteins that are no longer needed or damaged are disposed of in this way. Bacteria and viruses even allow themselves to be “eaten”, in order to enter and infect the cell.
The internalization of material is also called endocytosis. This process occurs in all cells and is absolutely vital. For a long time, Prof. Anne Spang’s team at the Biozentrum of the University of Basel has been studying how cells take up, sort, recycle and degrade the molecular cargo. “This often failed due to not being able to meet the technical requirements, needed to elucidate individual steps or the dynamics of the process in detail,” says Anne Spang. “For this problem, we have now found a simple and inexpensive solution.” The findings have been published in “eLife”.
Uptake and transport of cellular cargo
During endocytosis, the material to be ingested is engulfed by the cell membrane, which then buds off forming a vesicle. This filled vesicle fuses inside the cell with tiny organelles, the early endosomes. Here the cargo is sorted, some are sent back to the cell membrane, other components can be recycled or are unusable and must be degraded. All this occurs in the course of maturation from the early to the late endosome. The late endosomes ultimately fuse with the lysosome, which contains enzymes that digest the remaining waste. In this way, the cell constantly renews its membrane and simultaneously disposes of garbage.
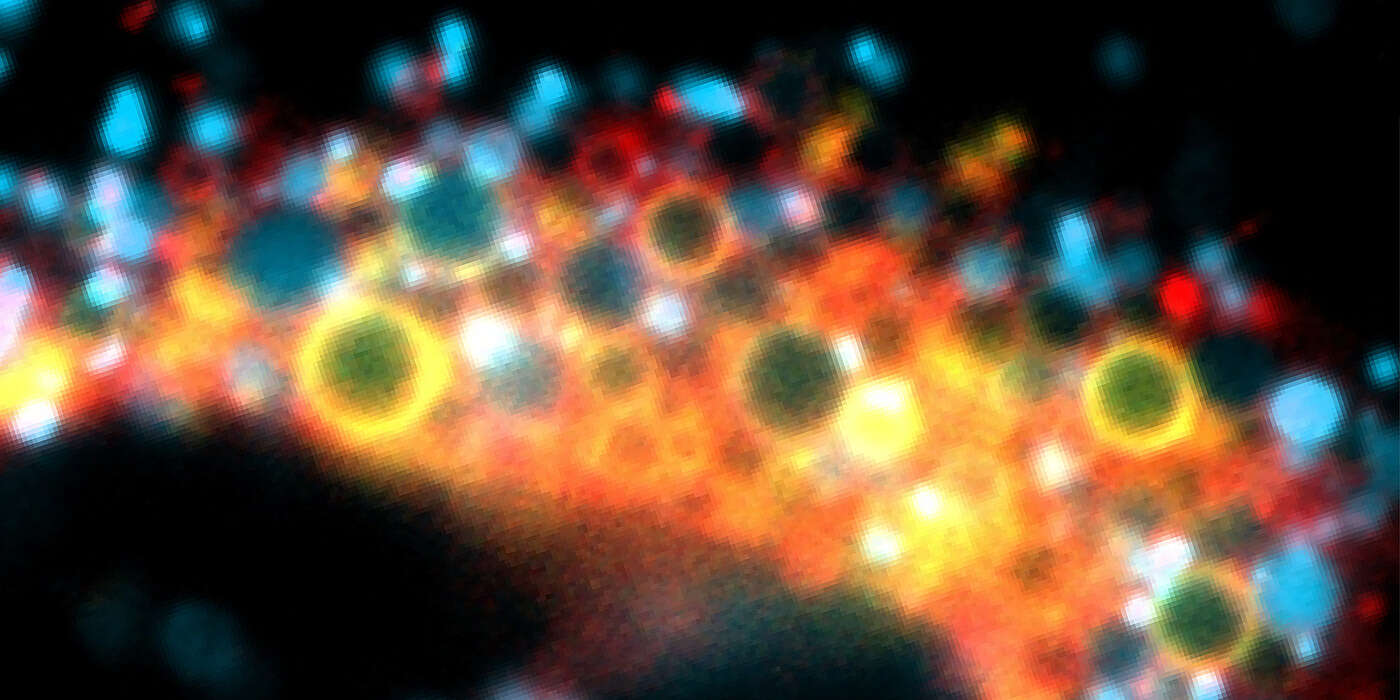
Enlarged endosomes visible under a microscope
The endosomes are some 200 to 250 nanometers in size, about as small as viruses. Their size makes it difficult to see and study them, as the resolution of conventional microscopes is not sufficient. “Previously, in order to observe the maturation and dynamics of the endosomes in more detail, access was required to technically sophisticated, super high-resolution microscopes,” says Spang. “We have now developed a method enabling us to artificially enlarge the endosomes. This makes them better visible, so that it is possible to observe them with a regular microscope without extra features.”
Stress induces formation of enlarged endosomes
In their assay, the researchers use a chemical substance to put the cells under stress, which respond by producing larger endosomes. To use this method for research, it was first important to verify whether the artificially enlarged endosomes behaved exactly as endosomes in non-treated cells. And indeed, they do go through the normal maturation cycle from early to late endosome to finally fuse with the lysosomes.
“With our method we have demonstrated for the first time that the maturation of endosomes is coupled to an increasing acidification of the internal environment,” says Spang. “We still don’t know how the two processes are coordinated but now we have a good system to look at this more closely.” In the future, Spang’s team plans to study the lysosomes in more detail, where non-recyclable debris, such as damaged proteins are digested. What happens to the cellular waste, how do the lysosomes change as they dispose of the waste, regenerate again and re-enter the cycle, are just a few open questions.
Original publication:
Maria Podinovskaia, Cristina Prescianotto-Baschong, Dominik P. Buser and Anne Spang. A novel live cell imaging assay reveals regulation of endosome maturation. eLife; published online 30 November 2021
Contact: Communications, Katrin Bühler