Each year, thousands of patients in Swiss hospitals become infected with dangerous pathogens that can hardly be controlled with antibiotics. The methicillin-resistant bacterium Staphylococcus aureus, MRSA for short, is particularly feared among the multi-resistant nosocomial germs. It can cause severe wound, respiratory and urinary tract infections and life-threatening sepsis. This is aggravated by the fact that MRSA causes chronic infections.
The cell wall as a therapeutic target
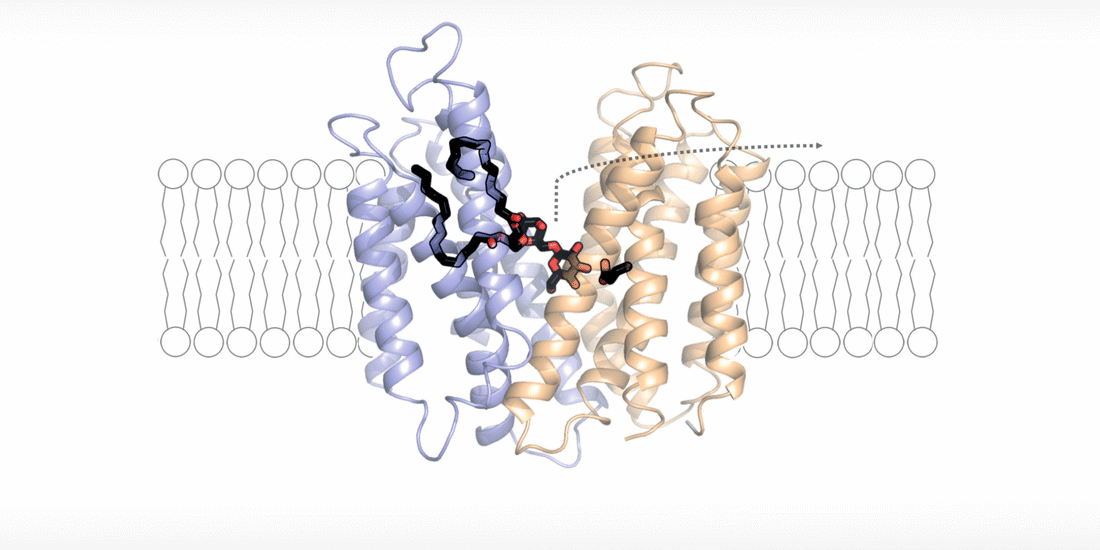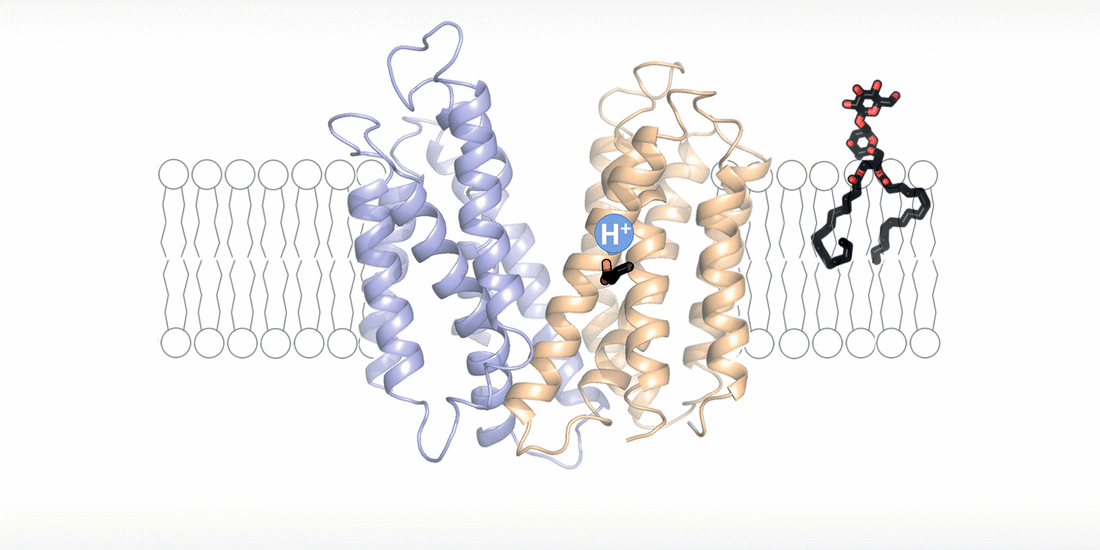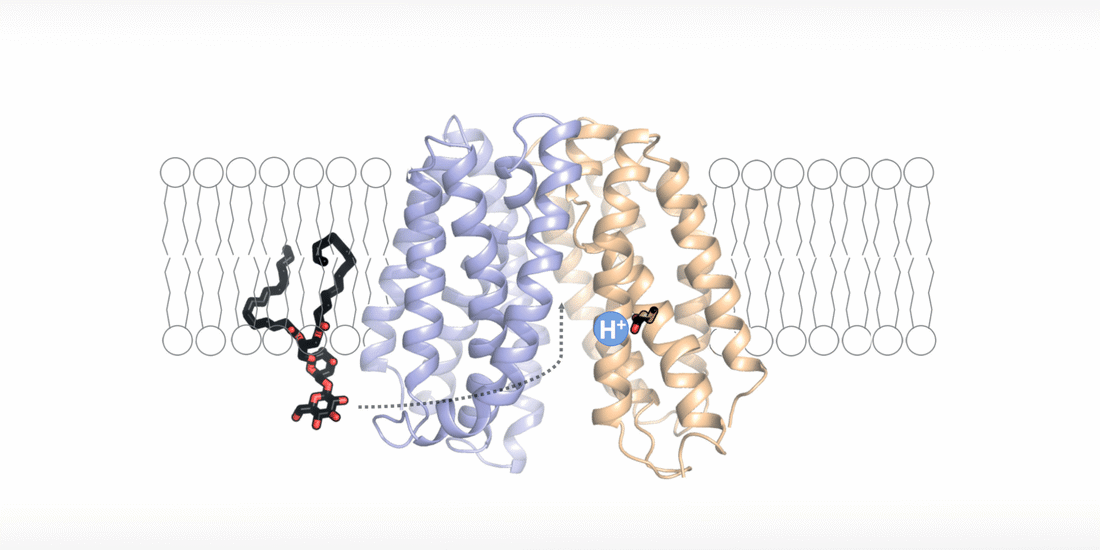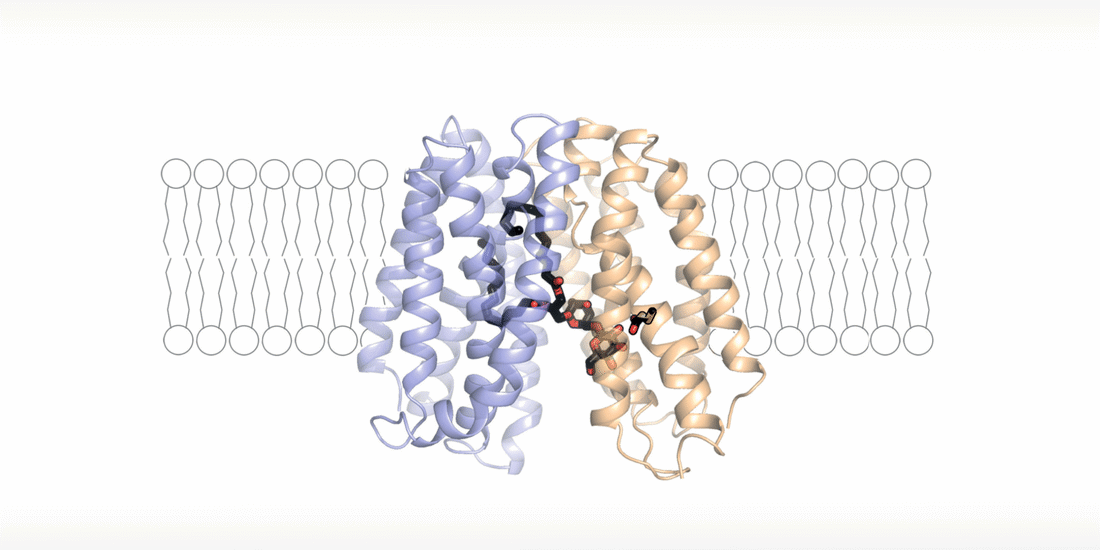
The bacterial cell wall is a key target in the search for new antimicrobials, as only an intact cell wall can protect the pathogens from the host's immune defence and from antibiotics. In a recent study, scientists led by Prof. Camilo Perez from the Biozentrum, University of Basel, have elucidated the structure and function of a flippase transporter involved in the synthesis of lipoteichoic acids in the pathogen MRSA. Lipoteichoic acids are important biopolymers that provide stability to the cell wall of Gram-positive bacteria, facilitate colonization of the host and contribute to repelling antibiotics.
Transport of an “anchor” molecule to its destination
The cell wall is a highly dynamic layer that surrounds the cell membrane and protects bacteria. Lipoteichoic acids are long-chain biopolymers that are embedded in the cell wall. However, they only remain in place because they are bound to an “anchor” molecule at the cell membrane. Without this “anchor”, lipoteichoic acids are not able to provide stability to the cell wall. "Based on our structural and functional analyses, we have been able to show for the first time how the “anchor” arrives at its destination and how bacteria energize this process," explains Perez. By moving hydrogen ions across the cell membrane, the flippase transporter is flipping the “anchor” molecule from the inside of the bacterial membrane, the site of its synthesis, to the outside, the site of lipoteichoic acid production.
Survival strategy of Gram-positive bacteria
"The fact that the transport of hydrogen ions is coupled with the synthesis of lipoteichoic acid represents a major survival advantage for these bacteria," says Perez. "The niches in the human body, which are preferentially colonized by Staphylococcus aureus, usually have an acidic microclimate. This means that the concentration of hydrogen ions is higher in these niches. The bacteria withstand these acidic conditions by simply building up a thicker protective layer of lipoteichoic acids."
The researchers have also been able to show that Staphylococcus aureus lacking the flippase transporter display severe growth defects upon acidic stress. According to the researchers, the flippase is essential for the survival of Staphylococcus aureus in our body and could be considered as a new pharmacological target for the treatment of dangerous MRSA infections.
Original article:
Bing Zhang, Xue Liu, Elisabeth Lambert, Guillaume Mas, Sebastian Hiller, Jan-Willem Veening and Camilo Perez. Structure of a proton-dependent lipid transporter involved in lipoteichoic acids biosynthesis. Nature Structural & Molecular Biology, published online 4 May 2020.
Contact: Communications, Katrin Bühler