Main Content
Understanding biomolecular function
Nuclear magnetic resonance methods provide unique insights into the structure, dynamics, and interactions of biomolecules.
All biological processes, for example cell division, infection or development of cancer, are based on specific interactions between biomolecules. These interactions are consequences of the atomic structures of biomolecules and their changes with time. Nuclear magnetic resonance (NMR) can determine both structure and dynamics of biomolecules and their complexes in solution. This makes it possible to understand biomolecular function at a fundamental level and also to develop strategies for interfering with function, e.g. by drugs.
Disease-relevant proteins
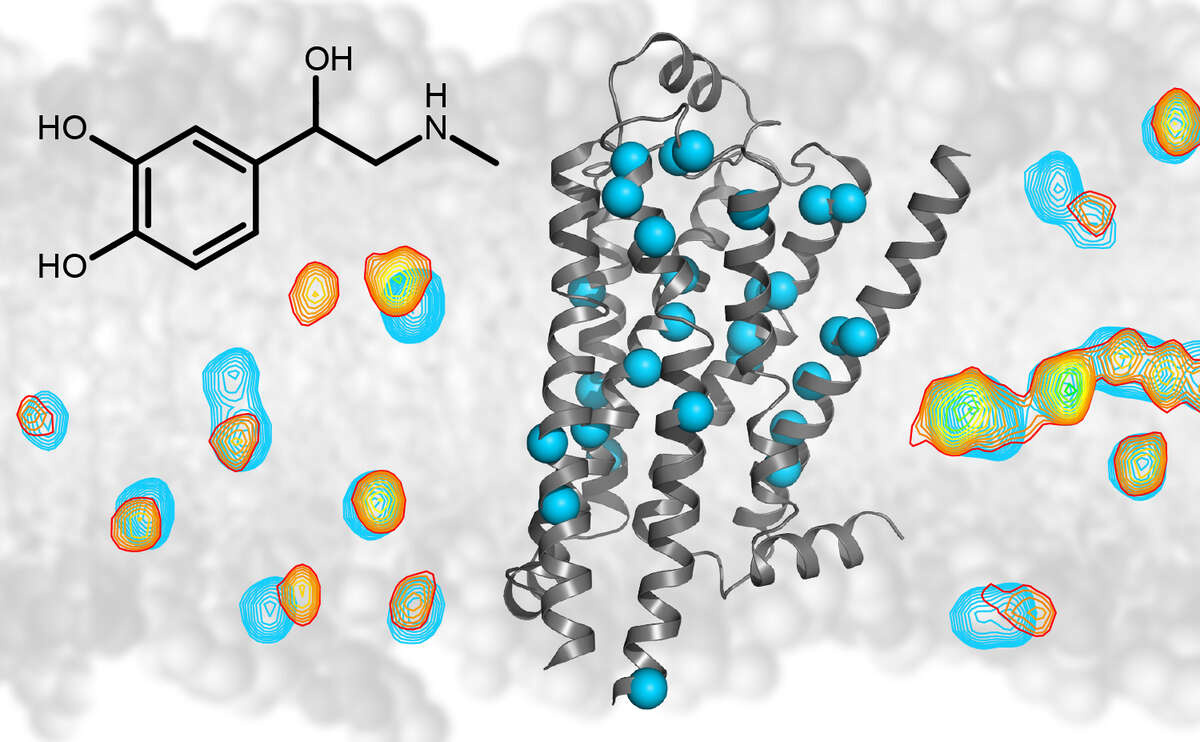
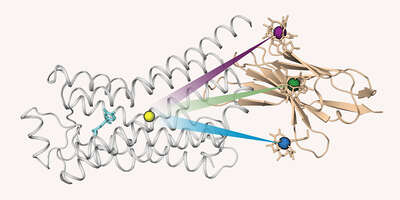
Our research is focused on problems in structural biology where NMR can yield unique new information on biomolecular structure, dynamics, and function. In particular, we are interested in molecules associated with human diseases, such as Abl kinase, a protein responsible for the development of chronic myeloid leukemia, the HIV-1 coreceptor CCR5, the β1-adrenergic receptor, or lipopolysaccharide, the molecule responsible for endotoxic shock.
Further development of NMR methods
A second focus is the development of new NMR methods that yield a better description of protein structure, dynamics and physicochemical interactions. In particular, we are developing new technologies for a highly quantitative description of unfolded protein states and of hydrogen bonds.