Main Content
Cell signaling and dynamics in bacterial growth, adaptation, and persistence
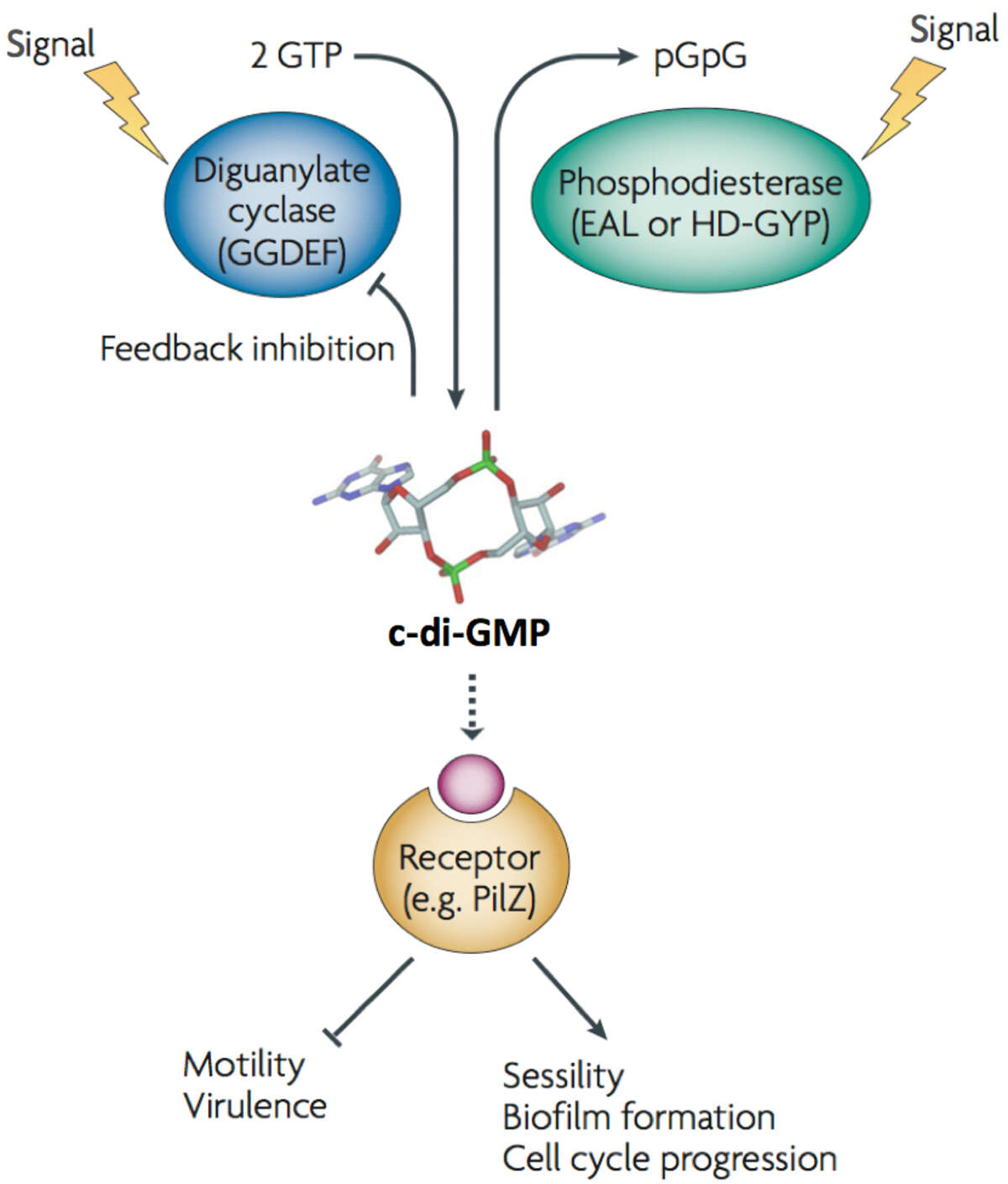
Our studies aim at understanding the molecular and cellular principles involved in the growth, differentiation and behavior of bacterial cells. We focus on the nucleotide second messenger, cyclic di-guanosine-monophosphate (c-di-GMP) and its role in bacterial cell signaling and dynamics (Schirmer & Jenal, 2009, Nature Reviews Microbiol 7, 724). C-di-GMP emerges as a ubiquitous signaling molecule that modulates multiple aspects of bacterial growth and behavior, including the formation of a sedentary, community-based lifestyle and its association with chronic forms of bacterial infections (Figure 1) (Böhm 2010 Cell, 141, 107; Malone 2012 PLoS Pathog., 8(6), e1002760; Steiner 2013 EMBO J, 32(3) :354). Our aims are to identify and characterize c-di-GMP control modules in different bacterial model organisms, to uncover and exploit the basic molecular and mechanistic principles of c-di-GMP signaling, and to probe its role in bacterial growth and persistence.
Role of c-di-GMP in cell cycle progression and cell fate determination
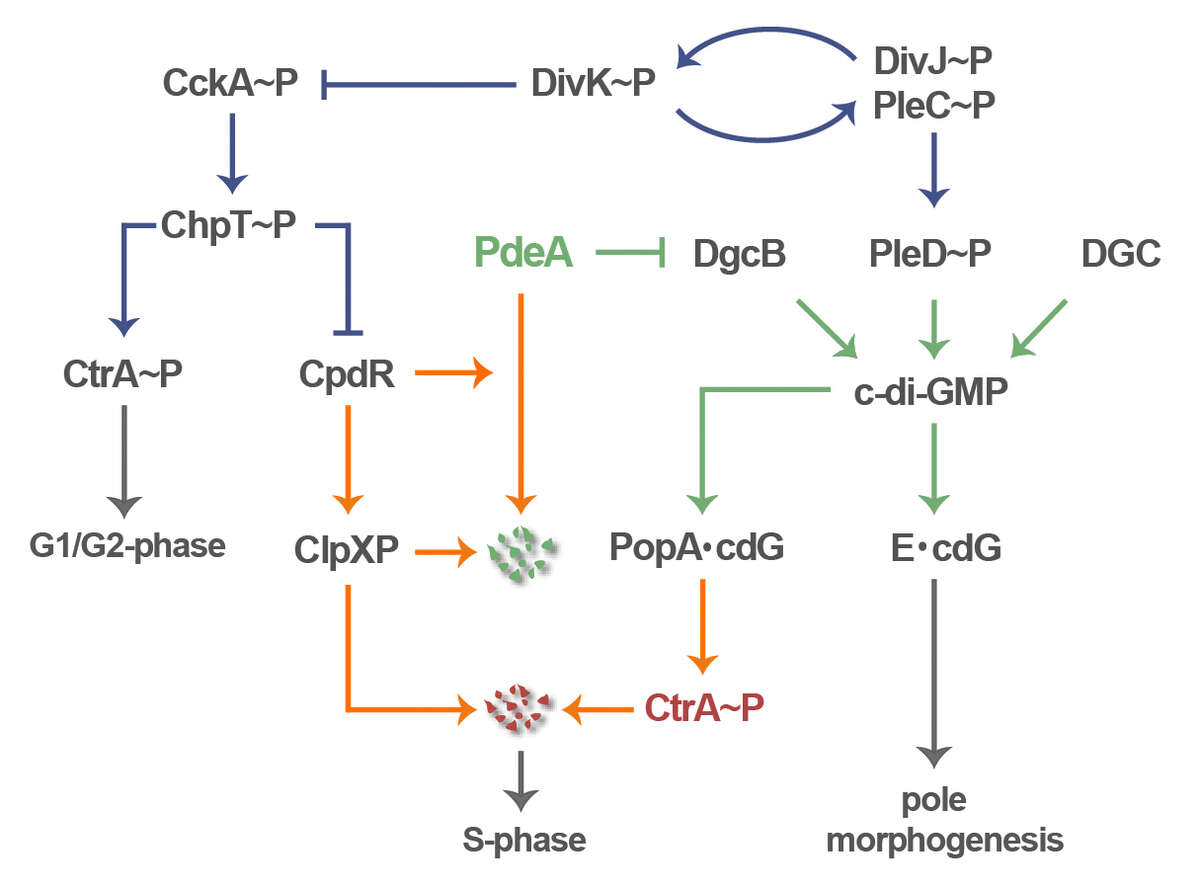
We use Caulobacter crescentus as a model to investigate the role of c-di-GMP in cell polarity and cell cycle progression. Periodic fluctuations of c-di-GMP are an integral part of the C. crescentus cell cycle clock and serve to control pole development in time and space and to coordinate these processes with the underlying cell cycle. Several diguanylate cyclases (DGC) and phosphodiesterases (PDE) contribute to the characteristic bimodal distribution of the second messenger during the Caulobacter life cycle. One of our aims is to understand their regulation in time and space (Abel 2013 PLoS Genet 9(9): e1003744). E.g. the DGC PleD is activated by phosphorylation prior to S-phase entry and sequesters to the differentiating Caulobacter cell pole where it orchestrates pole morphogenesis and replication initiation (Figure 2) (Paul 2008, Cell 133, 452, Abel 2011, Mol Cell 43, 550). Caulobacter G1-to-S transition is mediated by a second DGC, DgcB. In the G1 swarmer cell DgcB is “neutralized” by its specific and dominant antagonist PdeA, which reduces c-di-GMP in this cell type and keeps it in the motile, replication-inert phase. Upon entry into S-phase, when cells transit into sessility, PdeA is specifically degraded leaving DgcB unopposed. PdeA dynamically localizes to the old cell pole where it is degraded by the polar protease complex ClpXP. This cell cycle dependent process is orchestrated by the single domain response regulator CpdR, which itself localizes to the old cell pole in response to its phosphorylation status, where it recruits both PdeA and ClpXP and mediates substrate delivery (Abel 2011, Mol Cell 43, 550). Together, PDE degradation and DGC activation result in a rapid and robust upshift of c-di-GMP, which coordinately drives pole morphogenesis and S-phase entry (Abel 2011, Mol Cell 43, 550).
C-di-GMP controls all aspects of Caulobacter polarity, including flagellar motility, pili biogenesis as well as holdfast and stalk formation (Abel 2013 PLoS Genet 9(9): e1003744). The mechanisms and regulatory components contributing to timing and spatial control of polar organelle formation are one main focus of our current research (Davis 2013, Genes Dev 27, 2049). In parallel, we are interested in how c-di-GMP modulates Caulobacter cell cycle progression. The c-di-GMP up-shift facilitates replication and cell division control through the controlled destruction of the replication initiation inhibitor CtrA and the cell division inhibitor KidO by the ClpXP protease complex. Cell cycle dependent degradation of these proteins entails a specific spatial arrangement where both protease and substrates transiently localize to the incipient stalked cell pole during the G1-S transition. Substrate delivery to the polar protease requires PopA, a protein that sequesters to the stalked pole upon binding of c-di-GMP (Duerig 2009, Genes Dev 23, 93). Our recent studies demonstrate how phosphosignaling, protein degradation, and c-di-GMP mediated regulatory processes are tightly interconnected to coordinately drive the Caulobacter life cycle (Figure 2) (Abel 2011, Mol Cell 43, 550; Abel 2013 PLoS Genet 9(9): e1003744).
Role of c-di-GMP in biofilm formation and persistence
We have used Escherichia coli as a genetically versatile model organism to analyze the molecular basis of the inverse regulation of cell motility and biofilm formation by c-di-GMP. Our studies revealed that E. coli can fine-tune its swimming speed with the help of a molecular brake (YcgR) that, upon binding of c-di-GMP, interacts with the motor protein MotA to curb flagellar motor output (Böhm 2010 Cell, 141, 107). These experiments demonstrate that bacteria can modulate motor output in response to environmental cues. Our studies also led to identify c-di-GMP and ppGpp as key regulatory factors of poly-β-1,6-N-acetyl-glucosamine (poly-GlcNAc) synthesis, a polysaccharide adhesin secreted by E. coli as response to sub-inhibitory concentrations of antibiotics targeting the ribosome (Böhm 2009, Mol Microbiol. 72, 1500). The synergistic roles of ppGpp and c-di-GMP in biofilm induction, suggested that interference with bacterial second messenger signaling might represent an effective means for biofilm control during chronic infections.
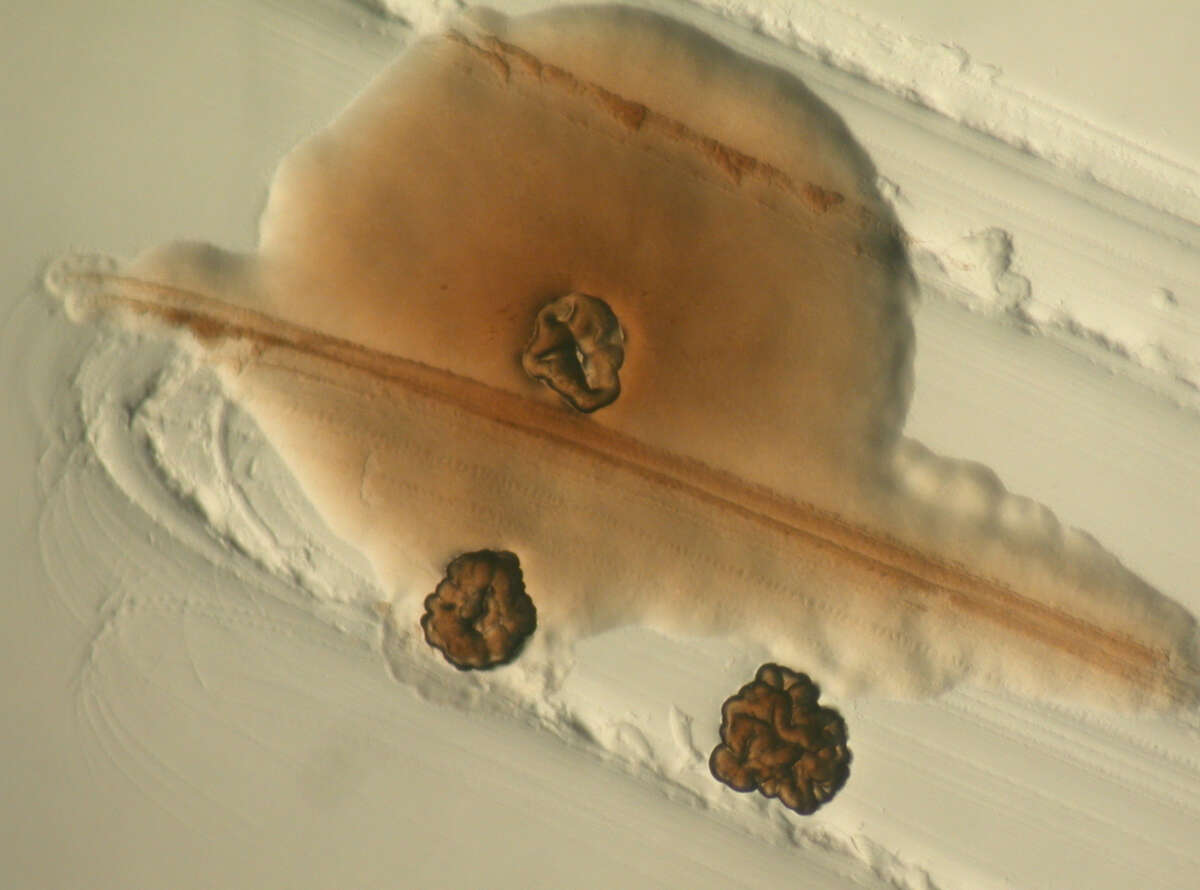
Chronic Pseudomonas aeruginosa infections in cystic fibrosis (CF) patients can be treated with antibiotics, however full clearance is not possible due to the adaptation of infective species to a persistent lifestyle. Adaptive P. aeruginosa morphotypes include small colony variants (SCVs), slow growing and strongly adherent variants whose appearance correlates with poor lung function (Figure 3). Our research on P. aeruginosa SCVs suggests that SCV-mediated persistence might be a novel target for antimicrobial chemotherapy. We characterized a tripartite signaling system called YfiBNR, mutations in which lead to the generation of SCV variants (Malone 2010, PLOS Pathogens, 6(3), e1000804). YfiN was shown to be a membrane-bound cyclic di-GMP synthase, whose activity is tightly controlled by YfiR and YfiB. Activation of YfiN resulted in increased levels of c-di-GMP, which in turn triggered massive production of exopolysaccharides, drastically reduced growth rates, and resistance to macrophage phagocytosis. Consistent with a role for the SCV phenotype in immune system evasion, activation of YfiN significantly increased the persistence of P. aeruginosa in long-term mouse infections. Moreover, the Yfi system is under positive and negative selection in airways of CF patients (Malone 2012, PLoS Pathogens, 8(6), e1002760) driving population dynamics of persistent SCVs in vivo. These studies establish a firm causal link between SCV, c-di-GMP, and chronic P. aeruginosa infections.
Important Partners
Martin Ackermann (ETH Zürich); Howard Berg (Harvard University); Alain Filloux (Center for Molecular Microbiology and Infection, Imperial College, London, UK); Stephan Grzesiek (Biozentrum, University of Basel); Tilman Schirmer (Biozentrum, University of Basel); Torsten Schwede (Biozentrum, University of Basel); Victor Sourjik (DKFZ-ZMBH, University of Heidelberg); Daniel Ritz (Actelion Pharmaceuticals Ltd., Allschwil, Switzerland); Volker Roth (Department of Computer Science, University of Basel); Patrick Viollier (Department of Microbiology and Molecular Medicine; University of Geneva); Jörg Vogel (Institute for Molecular Infection Biology, Würzburg University); Julia Vorholt (ETH Zürich); Michaela Zavolan (Biozentrum, University of Basel).