Main Content
Protein Bioinformatics in 3D: sequence – structure – function
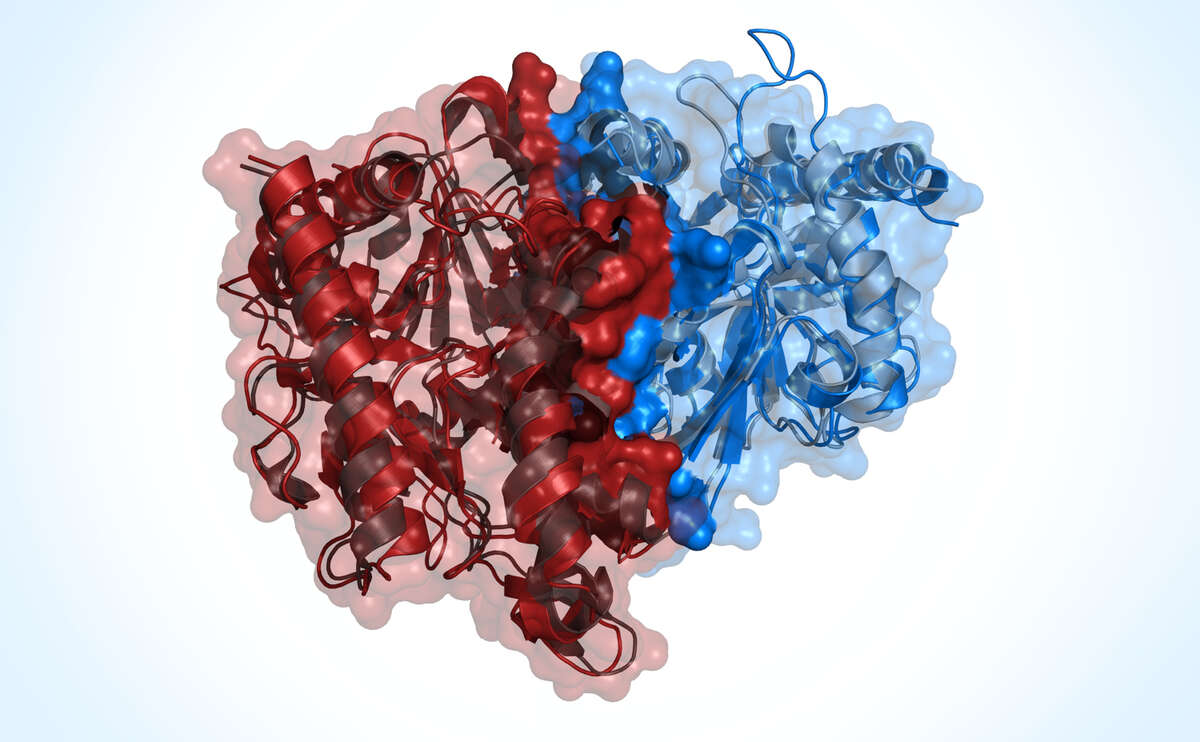
The function of proteins can be studied at the atomic level using methods for molecular modeling and simulation of their three-dimensional structures.
Proteins are the key tools of the cell and are responsible for the most important functions of living organisms. The specific spatial structures of proteins are decisive for their function. Computer simulations allow us to model the properties of three-dimensional protein structures and obtain information about their function at an atomic level of detail. This knowledge helps in basic research on protein function as well as in drug development.
Computational Modeling of 3D structures
We develop computer programs which allow us to model the structure of proteins that have not previously been elucidated experimentally. We are focusing on comparative protein modeling methods, also known as "homology modeling". By this technique, the structure of an unknown protein is interpolated from structures of known related proteins. Using this approach, reliable predictions can be made for most proteins in typical model organisms.
Which mutations cause disease?
The possible effects of mutations on the function of a particular protein can often be interpreted with the help of its structure. This knowledge makes it easier to understand hereditary diseases in humans and to interpret the variations observed in the human genome. Conversely, computer models can be used for targeted planning of mutations in proteins in order to investigate their function in the laboratory.
Specific interactions with small molecule
Many proteins show interactions with small chemical molecules, e.g. with metabolites during metabolism or hormones in signal transmission. Computer simulations allow a better understanding of the receptor's binding specificity. Structure-based drug development involves a targeted search for chemical molecules that specifically occupy a particular binding site.
Macromolecular assemblies
Cellular processes often depend on interactions between proteins and the formation of macromolecular complexes. Impairments of such interactions can lead to deregulation of pathways resulting in disease states. Detailed insight into such interactions is enabled by studying macromolecular models available with the latest SWISS-MODEL. Our method solely employs amino acid sequences of the interacting proteins as a user input to infer the stoichiometry and the overall structure of the assembly by homology modeling.