Main Content
Muscle aging
Rapid advances in treating life-threatening, age-related diseases, such as cancer and cardiovascular disease, have extended human lifespan in many developed countries. However, greater longevity has meant more people experience other age-related diseases, including sarcopenia, the age-related loss of muscle mass and strength. Maintaining healthy muscles as we age can make the difference between living independently and needing full time care.
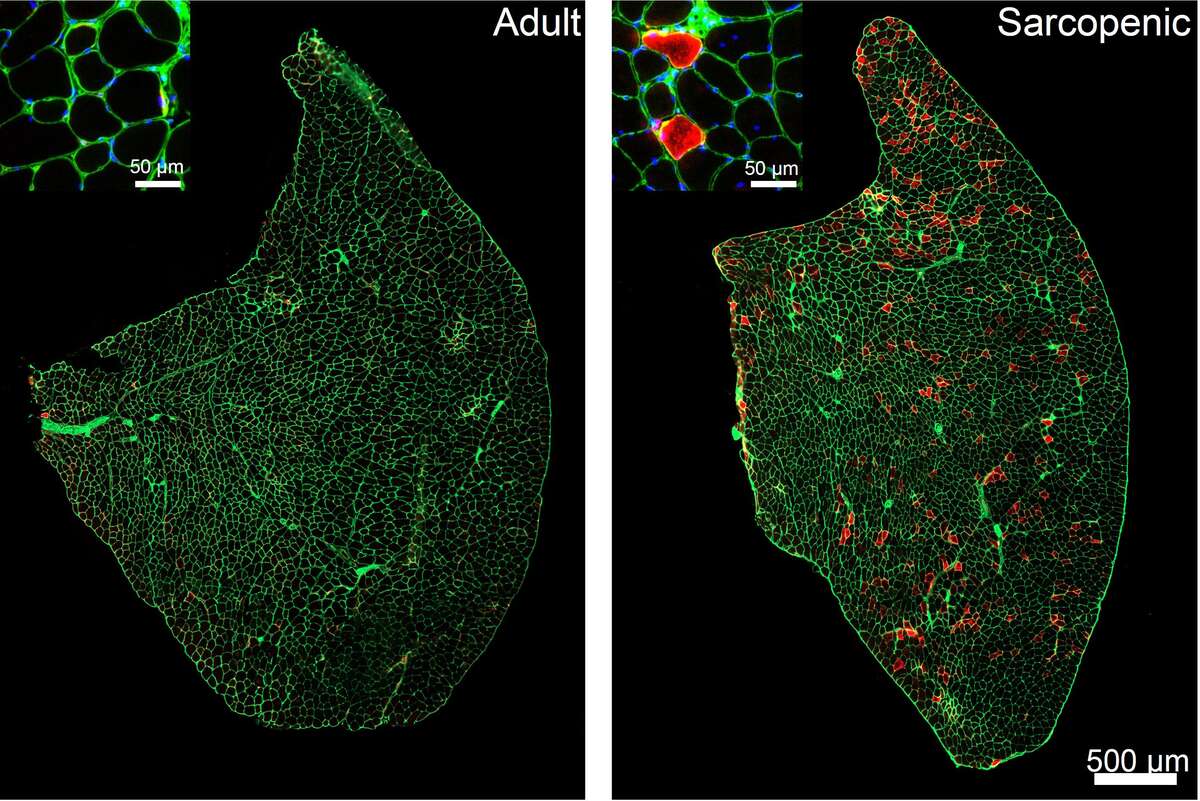
What causes our muscles to age and what can we do about it? By studying sarcopenia in wild-type and genetically modified mice we have found that hyperactivity of the mammalian target of rapamycin complex 1 (mTORC1) is one factor driving muscle aging and by suppressing its activity we can slow muscle aging. Below, you can see high mTORC1 activity (red staining) in sarcopenic muscle fibers.
The finding that high mTORC1 activity drives muscle aging is surprising, since our previous work highlights the importance of mTORC1 activity for muscle growth and development (Bentzinger et al., 2008; Rion et al., 2019). mTORC1 is a master regulator of protein homeostasis, integrating environmental cues, such as energy status, nutrient availability and growth factor levels, to control protein synthesis. Since skeletal muscle fibers are postmitotic and rely on changes in cell size, rather than cell number, activating mTORC1 is frequently touted as a strategy to counteract muscle aging. However, constant activation of mTORC1 in skeletal muscle does not cause muscle growth, but rather leads to a progressive, aging-like phenotype (Castets, Lin et al., 2013).
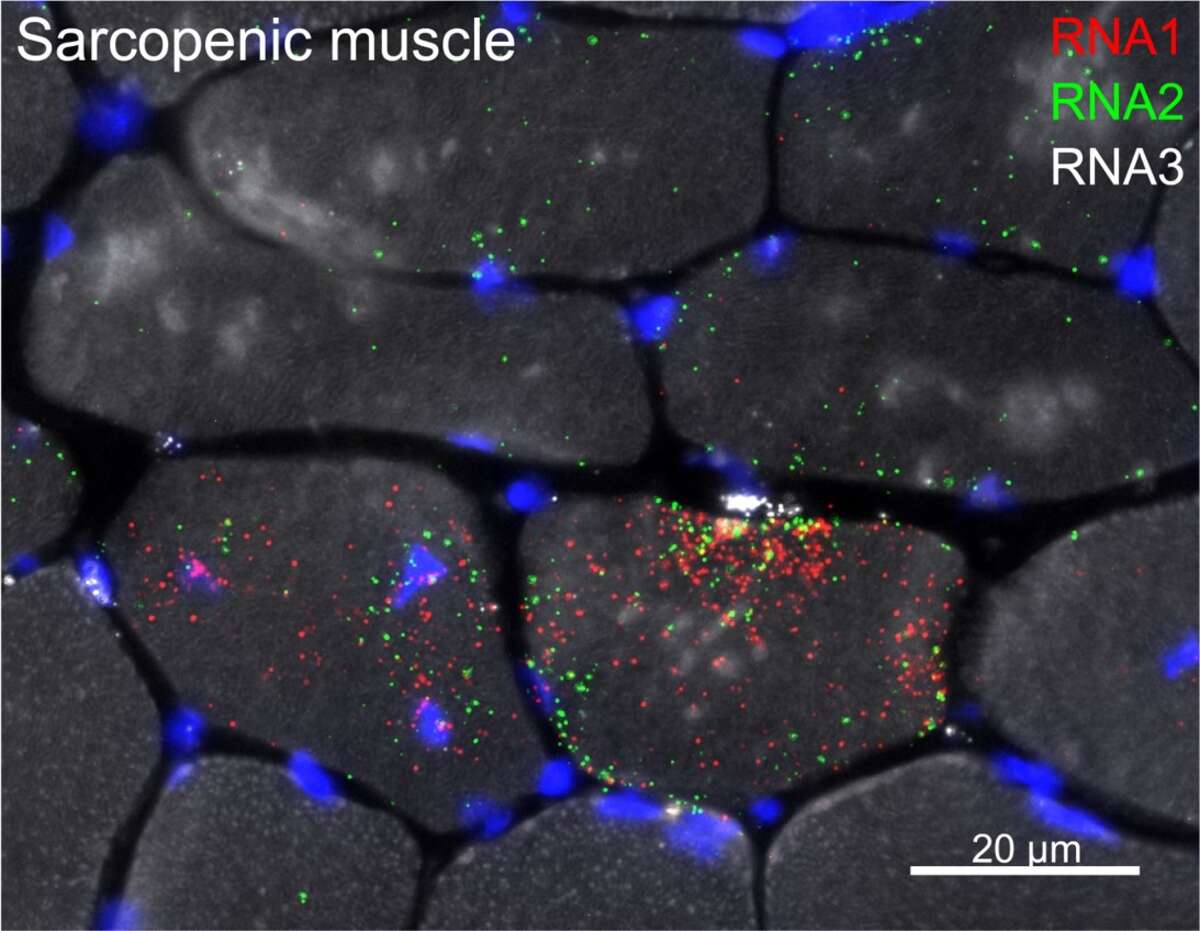
By combining transcriptomic profiling with models of delayed and precocious sarcopenia, we are aiming to further unravel the underlying mechanisms that make muscles age. There are many transcriptional changes in sarcopenic muscle, but what are the initiating stimuli and which cells are responsible remains unclear. Using the latest techniques for identification and visualization of transcriptional changes, we are now defining the spatial and temporal regulatory networks involved in muscle aging. Below, RNA Fluorescence In Situ Hybridization (FISH) shows distinct localization of sarcopenic signals in specific muscle fibers as well as in cells located between muscle fibers.
Funding:
SNSF
Novartis Research Fundation