In times of “home office”, lock downs and closed gyms, endurance sports is back in trend again. Jogging, biking or swimming keeps you healthy and makes your muscles fit. With regular training, the musculature adapts to be able to withstand strong workloads for longer. The protein PGC-1α is responsible for these adaptations. Together with the Professors Sebastian Hiller and Maria Hondele, Professor Christoph Handschin has now discovered how PGC-1α controls thousands of genes and thus prepares muscles to meet demands to resist fatigue.
Muscles change during endurance training
PGC-1α plays a key role in the muscle adaptation to endurance training. The protein initiates a genetic program which optimizes the energy output in muscle cells. For example, amongst many other changes, mitochondrial biogenesis increases. These power plants produce energy for the cells. Trained muscle fibers store large energy reserves in the form of sugars and fats and the tissue is supplied with more blood, thus increasing the supply of oxygen and further energy substrates. In addition, PGC-1α also stimulates muscle repair and regeneration.
The changes in the muscles are diverse and so too is the spectrum of genes triggering these changes. “We already knew that PGC-1α coordinates a complex genetic program. We have previously observed that inactive mice with increased PGC-1α expression develop muscles like their trained peers and can run for long even without training,” says Handschin. “However, it remained unclear how a single protein can regulate such a huge number of genes and recruit the necessary factors.”
PGC-1α droplets important for gene regulation
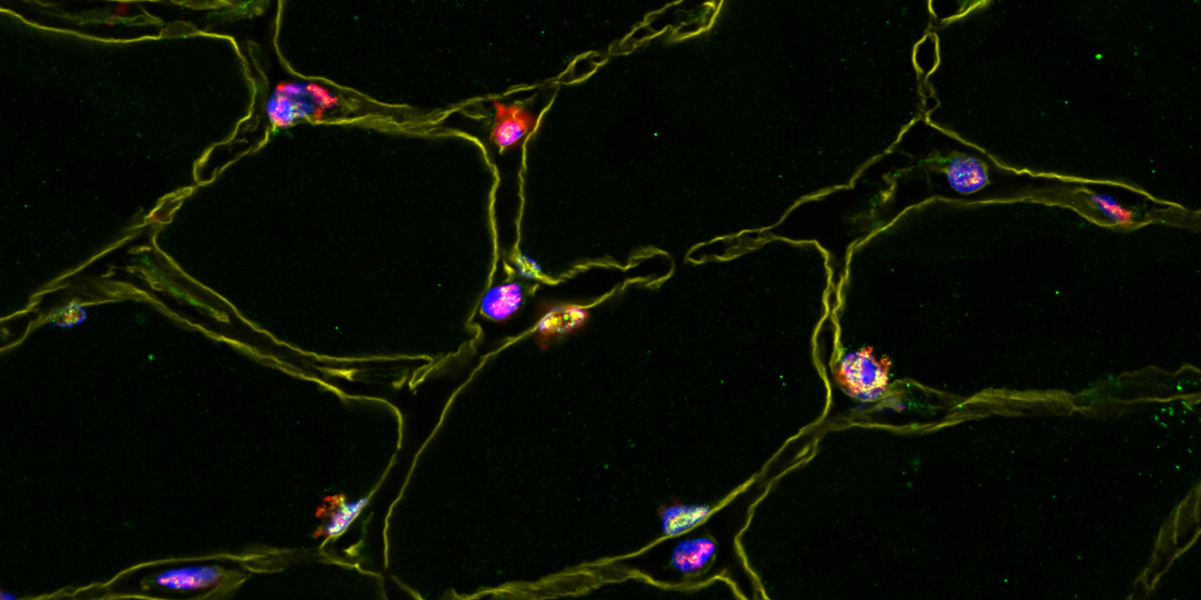
Using an animal model, the researchers have now been able to elucidate how PGC-1α controls gene expression in time and space. In the cell nucleus, the protein forms numerous tiny droplets together with RNA molecules and other proteins, similar to oil drops in water. “We could observe that these droplets enclose specific DNA regions and that the genes are actively transcribed at these sites,” explains Handschin. “Packed in these condensates, the set of molecules required for gene expression reach their specific destinations in the genome. We have also been able to detect these droplets in human muscles tissue.»
How is droplet content related to training adaptation?
Droplet formation in the cells is still an underappreciated but important process. It enables certain key regulators such as PGC-1 α to control and coordinate very complex biological programs, in this case muscle adaptation to training. As the droplets are found in trained as well as untrained muscles, the researchers suspect that depending on the fitness level, either the sites at which the droplets bind to DNA or the composition of the droplets change. Prof. Christoph Handschin and his colleagues plan to study these and further questions in the future.
Original publication:
Joaquín Pérez-Schindler, Bastian Kohl, Konstantin Schneider-Heieck, Aurel B. Leuchtmann, Carlos Henríquez-Olguín, Volkan Adak, Geraldine Maier, Julien Delezie, Thomas Sakoparnig, Elyzabeth Vargas-Fernández, Bettina Karrer-Cardel, Danilo Ritz, Alexander Schmidt, Maria Hondele, Thomas E. Jensen, Sebastian Hiller and Christoph Handschin. RNA-bound PGC-1α controls gene expression in liquid-like nuclear condensates. Proceedings of the National Academy of Sciences (PNAS); published online 31 August 2021
Contact: Communications, Katrin Bühler